US Pharm. 2023;48(9):HS12-HS16.
ABSTRACT: Postpartum depression (PPD) is a debilitating form of perinatal depression that is diagnosed after childbirth. Symptoms are more severe and enduring than those of the baby blues, which is a common and short-lived condition involving mild depressive symptoms. Clinical-practice guidelines recommend that psychotherapeutic measures be used first-line when possible; however, the severity of symptoms must be considered in selecting a treatment method, as the benefits of pharmacotherapy may outweigh the risks, especially in more severe cases of PPD. Two agents are currently approved specifically for the treatment of PPD. Through education and counseling services and therapeutic interventions, pharmacists are essential in ensuring that medications are used appropriately in perinatal and postpartum patients in both outpatient and hospital settings.
It is common for new mothers to experience emotional changes after giving birth.1 They may also experience transient depressive symptoms attributable to a variety of factors, including hormonal fluctuations, lack of sleep, and challenges associated with adapting to a new lifestyle and unfamiliar responsibilities. This manifestation of mild depressive symptoms, often referred to as the “baby blues,” typically begins within a few days following delivery and resolves within 2 weeks.1 Postpartum depression (PPD) is a severe, debilitating form of perinatal depression that is diagnosed within the first year following childbirth, and its symptoms are distinct from those of the baby blues. Preexisting mental-health conditions increase the risk of depression onset after delivery.1-3 PPD is associated with negative outcomes for both newborn and mother, including failure to thrive and/or cognitive delays in the newborn, disrupted familial and social dynamics, difficulty bonding with the baby, and suicidality.3
According to the American College of Obstetricians and Gynecologists (ACOG)’s current guideline and a recent CDC report, preventable mental-health conditions are the most common cause of death in pregnant and postpartum women, with suicide and substance overdose reponsible for the most fatalities.3,4 Maternal suicide is a more frequent cause of postpartum mortality than postpartum hemorrhage or hypertensive disorders.4 In order to determine an effective treatment plan for PPD, whether on an outpatient or an inpatient basis, appropriate evidence-based screening methods must be implemented for conditions such as suicidal ideation and depression.3
Epidemiology and Etiology
PPD, which most commonly manifests within 6 weeks after childbirth, occurs in an estimated 6.5% to 20% of women (more than one in five).2,5 The prevalence of PPD is higher among adolescent mothers, women living in urban areas, and those who deliver prematurely than it is among other postpartum women. One study reported that African American and Hispanic women experienced PPD symptoms within 14 days of giving birth, whereas white women developed PPD symptoms after 14 days.5 Compared with other peripartum and postpartum women, African American women have been found to have an increased rate of suicidal ideation. Pregnant women are more likely than postpartum women to experience anxiety, and because comorbid anxiety and depression tend to occur perinatally, patients should be monitored throughout the perinatal and postpartum periods.3
Clinical Presentation and Diagnostic Criteria
Symptoms of PPD mimic those of major depressive disorder (MDD)—which is unrelated to childbirth—and may include the following: depressed mood, anhedonia, self-isolation from others, frequent crying, changes in eating and sleeping patterns, or having harmful thoughts toward oneself or others. Additional symptoms that are specific to PPD may include feelings of guilt or worthlessness as a mother, harmful thoughts toward the baby, and apathy when interacting with the baby.1,6
PPD is diagnosed using the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision, criteria for MDD.6,7 To receive a diagnosis of PPD, a patient must be within the postpartum period mentioned above and meet three of the specified criteria for a major depressive episode (TABLE 1). New mothers who experience depressive symptoms for 2 weeks or longer should report these symptoms to a medical professional because an evaluation for PPD may be warranted. Additionally, because some new mothers may develop PPD as late as 1 year following childbirth, it is recommended that regular clinical monitoring for depressive symptoms be performed.1,3

One important aspect of PPD diagnosis is the assessment of a patient’s risk of self-harm. It is recommended that patients who present with thoughts of self-harm be evaluated for the likelihood of a future suicide attempt. Thorough screening for symptom severity can clarify whether inpatient or outpatient treatment is necessary. Treatment for patients with a mild-to-moderate risk of self-harm typically can be managed in an outpatient setting, but patients who are at severe risk for self-harm may require inpatient management.3 Some indicators of high suicidal risk include feelings of hopelessness, an established plan, a timeline for carrying out the plan, sleeping less than 2 or 3 hours per night, and a lack of protective factors (e.g., children, religious beliefs, family). Depending on the screening and diagnostic results, both hospitalized and outpatient PPD patients can receive nonpharmacologic and pharmacologic therapies tailored to their specific treatment goals.2,3
Clinical Management
Although the American Psychiatric Association has a clinical practice guideline for depression management, the guideline was last updated in 2010.8 The ACOG clinical practice guideline, which was updated in 2023, provides more specialized information on treatment and management that is relevant for women who have PPD.2,8 It is recommended that nonpharmacologic psychotherapeutic measures, such as cognitive-behavioral therapy, be used first-line when possible; however, the severity of symptoms must be considered in selecting a treatment method, as the benefits of pharmacotherapy may outweigh the risks, especially in PPD cases that are more severe.2,8,9
Because unmanaged PPD symptoms can potentially result in poor outcomes, such as suicide, patients may require pharmacologic therapy. The ACOG guideline recommends that pharmacotherapy be used as first-line therapy in patients who are unlikely to respond to psychotherapy alone.2 For example, effective psychotherapy may be more difficult to provide to pregnant and postpartum patients who do not speak English, are uninsured, or live in a rural location. Additionally, pregnant and postpartum patients who have not responded to past psychotherapy sessions, lack access to psychotherapy, or are unlikely to partake in available psychotherapy options may require further pharmacologic management of their symptoms; therefore, it is recommended that pharmacotherapy be initiated as first-line treatment for depression in these patients.2
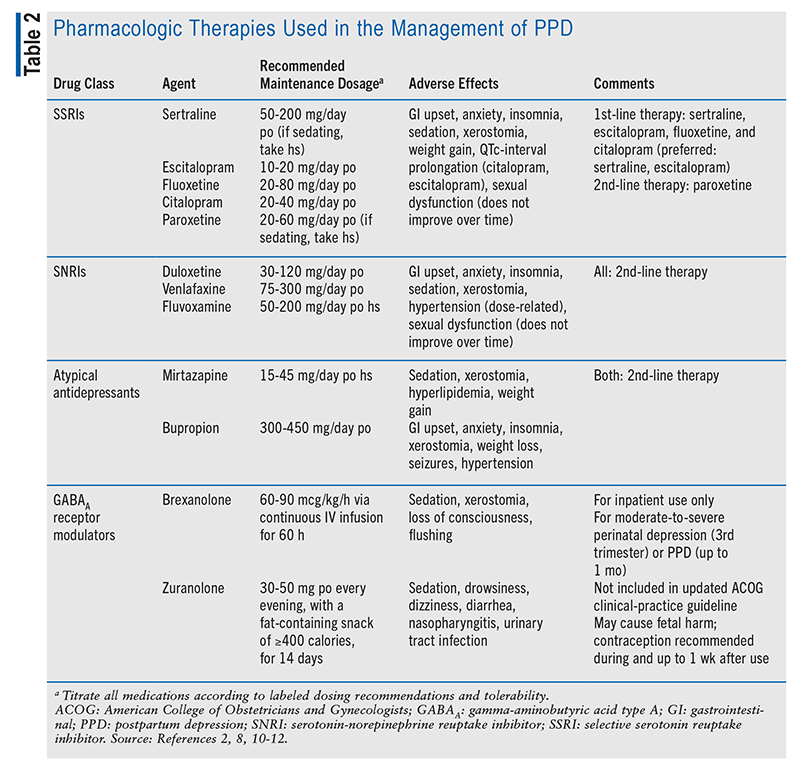
Selective serotonin reuptake inhibitors (SSRIs) are recommended as first-line pharmacologic therapy for perinatal depression and PPD because of their general tolerability and efficacy.2,8 The ACOG guideline considers sertraline and escitalopram to be the preferred SSRIs for initiation in treatment-naïve patients, as they have been associated with fewer instances of pulmonary hypertension in neonates compared with other antidepressants.2 However, if a patient is currently taking or has previously demonstrated tolerance and success with a different antidepressant, it is recommended that the patient continue on that agent to avoid having to cross-taper from one drug to another; this is because cross-tapering increases the potential for adverse effects in the neonate. The dosage should be titrated as tolerated before a medication change is attempted.2
Unlike other SSRIs, paroxetine is considered second-line therapy.2 Neonatal adaptation syndrome has been associated with paroxetine use during pregnancy; however, fluoxetine may also result in this adverse effect. Although there is an increased risk that the infant will develop pulmonary hypertension, this can also occur with other SSRIs. Therefore, patients who are already stabilized on paroxetine should not discontinue therapy during pregnancy or while breastfeeding, as it is preferable for the patient to continue an effective and well-tolerated regimen than to switch to another antidepressant, especially during the perinatal or postpartum period.2
Serotonin-norepinephrine reuptake inhibitors (SNRIs) have demonstrated efficacy in treating perinatal depression and PPD, but they are considered second-line therapy given the increased risk of preeclampsia and spontaneous abortion. Other antidepressants, such as mirtazapine and bupropion, may also be used as second-line therapy. In the selection of an agent, the potential for worsening anxiety must be considered because antidepressants that are stimulating (i.e., energizing) can contribute to anxiety symptoms. Bupropion and fluoxetine are two notable examples of stimulating antidepressants, but some other SSRIs and SNRIs also have energizing effects. A patient-specific approach to selecting pharmacotherapy that takes into account individual responses and reactions to medications should be employed.2
Brexanolone, a Schedule IV gamma-aminobutyric acid type A (GABAA) receptor modulator that is administered IV via continuous infusion over a 60-hour period, is FDA approved for the treatment of moderate-to-severe PPD.2,10 The ACOG guideline recommends the use of brexanolone in patients who are in the third trimester of pregnancy or within their first month postpartum.2 Brexanolone has a Risk Evaluation and Mitigation Strategies (REMS) program because of the risk of sedation; therefore, it must be administered in the hospital setting so that continuous pulse oximetry monitoring can be conducted.2,10 Additionally, patients must stop breastfeeding for 4 days after the end of the 60-hour infusion period. One advantage of brexanolone use is that symptom improvement can occur within 48 hours of administration, compared with 1 to 2 months for oral antidepressants.2
In August 2023, the FDA approved zuranolone (Zurzuvae), an oral GABAA receptor modulator, for the treatment of PPD in adult women.11,12 This is the first oral therapy for PPD that can be used in an outpatient setting. In clinical trials, symptom improvement was shown after 14 days of use. This agent carries a boxed warning for central nervous system depression, so it is recommended that patients refrain from driving or operating machinery for at least 12 hours after administration. Because of its abuse potential, the Drug Enforcement Administration is evaluating zuranolone for appropriate controlled-substance scheduling.11,12
TABLE 2 describes the recommended pharmacologic therapies for PPD.
The Pharmacist’s Role
Pharmacists are particularly valuable members of the healthcare team in the management of PPD symptoms, as they can improve outcomes by providing accurate and relevant patient education, evaluating therapeutic efficacy, and monitoring for adverse events.13 As experts in pharmacology, pharmacists can effectively counsel outpatients and inpatients with PPD on their medications. Because SSRIs are first-line therapy, pharmacists should provide patients with comprehensive education on these agents. Notable adverse effects to discuss include gastrointestinal upset, xerostomia, sleep disturbances, and sexual side effects. Although most of these side effects are transient, patients should be informed that sexual side effects may continue for the duration of therapy. Patients taking citalopram or escitalopram should be counseled about the increased risk of QTc-interval prolongation, especially with long-term use. Because it may take up to 2 months for SSRIs to demonstrate efficacy, it is critically important to advise patients on when to expect therapeutic efficacy.2
It is especially important for pharmacists to provide thorough patient education on brexanolone, as its REMS program requires that patients be informed of the risks of loss of consciousness and excessive sedation.13,14 Pharmacists can also conduct impactful nonpharmacologic counseling and educational interventions. One prospective observational study demonstrated the importance of involving a clinical pharmacist in the management of PPD: When clinical pharmacists provided supportive therapy to mothers with PPD, there was a reduction in the number of mothers who reported depressive symptoms within 1 month post intervention.13
Pharmacists are responsible for ensuring that medications are appropriately ordered and dispensed in inpatient settings; they also must be involved in predicting and communicating drug interactions.14 To dispense brexanolone to patients, pharmacists and pharmacies must be enrolled in the REMS program. Additionally, pharmacists should train other healthcare professionals to properly monitor patients receiving brexanolone for signs and symptoms of excessive sedation and loss of consciousness. Pharmacists must also ensure that the policies and procedures required by brexanolone’s REMS program are upheld.14 Given that brexanolone is a Schedule IV controlled substance and zuranolone is pending scheduling, the documentation of shipments and invoices is especially important.10,12,14
Conclusion
PPD is a debilitating condition that requires a multimodal approach to screening and treatment. Because of the potential for poor outcomes resulting from untreated PPD, the involvement of an interdisciplinary management team is necessary. The treatment of PPD should be individualized and specific to the patient. A number of nonpharmacologic and pharmacologic interventions are available for the management of PPD. Pharmacists can make a significant impact on PPD management in the inpatient setting by supplying optimal therapy and monitoring recommendations to the rest of the interdisciplinary team and by ensuring that patients are appropriately counseled on the medications prescribed for the management of PPD. The counseling services and therapeutic interventions pharmacists provide to inpatients and outpatients experiencing PPD, as well as the education they provide to healthcare professionals, are just some of the examples of how essential a resource pharmacists are for both the healthcare profession and the community.
REFERENCES
1. U.S. Department of Health & Human Services Office on Women’s Health. Postpartum depression. www.womenshealth.gov/mental-health/mental-health-conditions/postpartum-depression. Accessed June 16, 2023.
2. Treatment and management of mental health conditions during pregnancy and postpartum: ACOG Clinical Practice Guideline No. 5. Obstet Gynecol. 2023;141(6):1262-1288.
3. Screening and diagnosis of mental health conditions during pregnancy and postpartum: ACOG Clinical Practice Guideline No. 4. Obstet Gynecol. 2023;141(6):1232-1261.
4. Trost S, Beauregard J, Chandra G, et al. Pregnancy-Related Deaths: Data from Maternal Mortality Review Committees in 36 US States, 2017–2019. Atlanta, GA: CDC; 2022.
5. Mughal S, Azhar Y, Siddiqui W. Postpartum depression. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan-.
6. Burval J, Kerns R, Reed K. Treating postpartum depression with brexanolone. Nursing. 2020;50(5):48-53.
7. Coryell WH, Beach SR, Leibenluft E, et al. Depressive disorders. In: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR). Washington, DC: American Psychiatric Association Publishing; 2022:177-214.
8. Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice guideline for the treatment of patients with major depressive disorder, 3rd ed. American Psychiatric Association. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Accessed June 14, 2023.
9. American Psychiatric Association. Treating major depressive disorder: a quick reference guide. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd-guide-1410457165213.pdf. Accessed June 19, 2023.
10. Zulresso (brexanolone) product information. Cambridge, MA: Sage Therapeutics, Inc; June 2022.
11. FDA. FDA approves first oral treatment for postpartum depression. www.fda.gov/news-events/press-announcements/fda-approves-first-oral-treatment-postpartum-depression. Accessed August 8, 2023.
12. Zurzuvae (zuranolone) product information. Cambridge, MA: Biogen Inc and Sage Therapeutics, Inc; August 2023.
13. Hadia R, Bhavsar K, Patel R, et al. A prospective observational study to find out the characteristics of postpartum depression (PPD) and to assess the impact of pharmacist led counselling among the females with PPD. J Young Pharm. 2022;14(2):214-220.
14. FDA. Approved Risk Evaluation and Mitigation Strategies (REMS). Zulresso (brexanolone).www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=387. Accessed June 28, 2023.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






