US Pharm. 2020;45(4):16-20.
ABSTRACT: In 2019, guidelines for the management of immunocompetent adults with community-acquired pneumonia (CAP) were published jointly by the American Thoracic Society and the Infectious Diseases Society of America. Different treatment regimens are recommended depending on whether the patient is receiving treatment in the inpatient or outpatient setting, the severity of the pneumonia, and whether the patient has comorbidities or any risk factors for drug-resistant pathogens. The recommended duration of antimicrobial treatment for patients with CAP is a minimum of 5 days. Pharmacists should work actively with physicians to recommend appropriate antimicrobial therapy for the treatment of patients with CAP and are in a key position to ensure that patients receive the appropriate duration of therapy.
Community-acquired pneumonia (CAP), an infection of the lung parenchyma that occurs in persons outside of a hospital setting, is associated with high morbidity and mortality.1 In 2016, pneumonia was the primary diagnosis in more than 1.7 million patient visits to emergency departments in the United States.2 A recent study projected that CAP results in 1.5 million hospitalizations of adults in the U.S. annually, with about one in three CAP patients dying within 1 year.3 In 2019, in order to foster timely and accurate treatment, the Infectious Diseases Society of America and the American Thoracic Society jointly published guidelines for the management of immunocompetent adults with CAP.4 This article presents an overview of these guidelines and reviews the etiology of CAP and antimicrobial treatment options in both the outpatient and the inpatient setting.
Etiology and Diagnosis
The most common bacterial causes of CAP are Streptococcus pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae, Staphylococcus aureus, Legionella species, Chlamydia pneumoniae, and Moraxella catarrhalis. Although viral pathogens are becoming an increasingly common cause of CAP, the new guidelines recommend that all patients with CAP be treated empirically for bacterial infection. The basis for this recommendation is that no rapid and specific diagnostic test exists to confirm that a patient’s illness is due solely to a virus at the time of presentation, and patients with CAP caused by a virus often have a bacterial coinfection. Notably, the guidelines have eliminated the term healthcare-associated pneumonia, instead emphasizing the use of local epidemiology and risk factors to determine the need for coverage of drug-resistant pathogens, such as methicillin-resistant S aureus (MRSA) and Pseudomonas aeruginosa.4
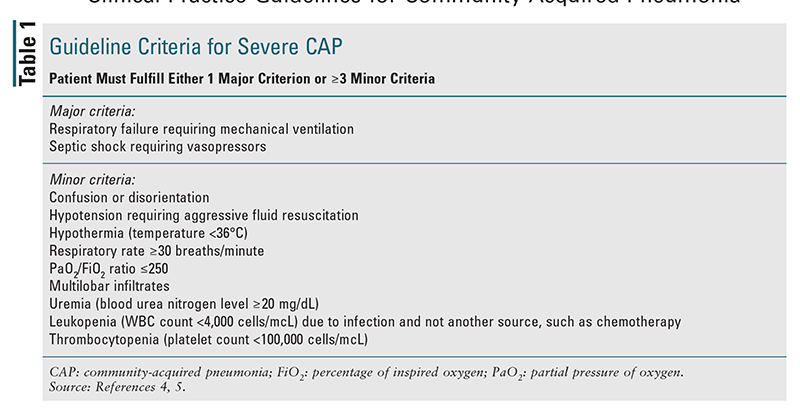
The updated guidelines do not recommend routine collection of sputum Gram stain and culture or blood cultures in CAP patients who are treated in the outpatient setting. In the inpatient setting, it is recommended that pretreatment sputum Gram stain with culture and blood cultures be obtained only in patients with severe CAP (as defined in TABLE 1), those being empirically treated for MRSA or P aeruginosa, and those with strong risk factors for these pathogens (described in more detail below).4,5 Testing for pneumococcal urinary antigen and Legionella urinary antigen is recommended for inpatients suspected of having severe CAP; testing for Legionella is also recommended if epidemiologic factors (e.g., an outbreak) suggest the possibility of infection. In both the inpatient and the outpatient setting, testing for influenza is recommended when influenza viruses are circulating in the community. The use of procalcitonin is not recommended to determine the need for initial antibiotic therapy in patients with CAP, and empirical antibiotic therapy should be initiated in adults with clinically suspected and radiographically confirmed CAP regardless of serum procalcitonin levels.4
Empirical Antibiotic Treatment
The guidelines recommend different treatment regimens for patients with CAP depending on the treatment location (inpatient or outpatient), whether the pneumonia is classified as severe according to the criteria in TABLE 1, and whether the patient has comorbidities or any risk factors for drug-resistant pathogens. Risk factors for MRSA and P aeruginosa include prior respiratory isolation of the pathogen or hospitalization with receipt of parenteral antibiotics within the past 90 days, with locally validated risk factors for these pathogens. According to the guidelines, the process of local validation involves obtaining local data on the prevalence of MRSA or P aeruginosa in CAP patients and determining risk factors for infection with these pathogens at a local, systemic level. The guidelines acknowledge that local prevalence data may not be known at all sites, so it is encouraged that sputum and blood cultures be obtained when risk factors for these pathogens are present, in order to generate local data.4
Any patient with CAP who was recently exposed to one class of antibiotics should be treated with an antibiotic regimen involving a different class.4 Additional considerations in choosing an empirical regimen for a patient with CAP include existing patient allergies, comorbid disease states, and adverse effects. Both macrolides and fluoroquinolones have QT prolongation listed under the prescribing information’s warnings and precautions, and fluoroquinolones have several additional warnings, including aortic aneurysm, tendinitis or tendon rupture, peripheral neuropathy, and central nervous system effects.6,7
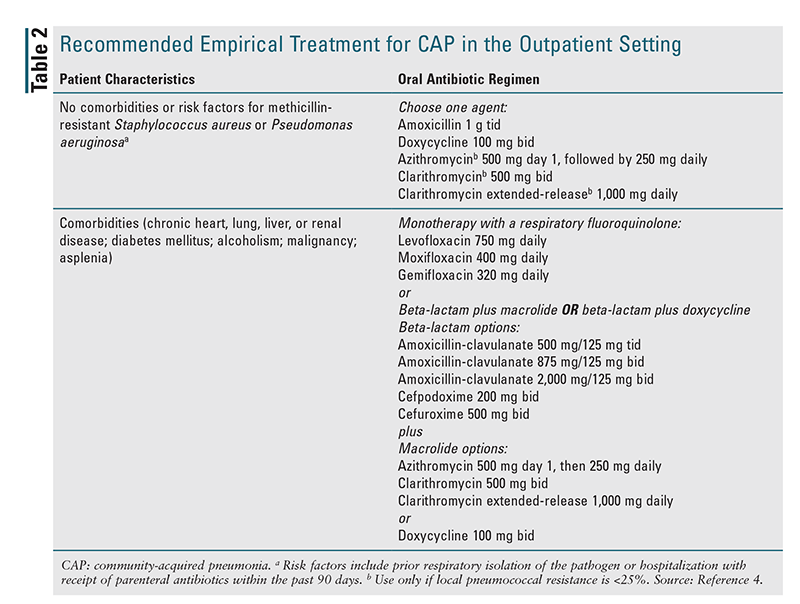
Outpatient Setting: Recommended empirical treatment for CAP in the outpatient setting is given in TABLE 2.4 For patients without comorbid conditions or risk factors for drug-resistant pathogens, monotherapy with amoxicillin, doxycycline, or a macrolide (azithromycin or clarithromycin) is recommended. However, owing to resistance-related factors, macrolide monotherapy went from being a strong recommendation in the previous guidelines to a conditional recommendation in the new guidelines.4 A macrolide should not be used if the local rate of resistance of pneumococcus is greater than 25%, and in the U.S., the average rate of pneumococcal resistance to macrolides is about 30%.5,8 For patients with comorbidities, the guidelines recommend broader-spectrum coverage consisting of either monotherapy with a respiratory fluoroquinolone (levofloxacin, moxifloxacin, or gemifloxacin) or combination therapy with amoxicillin-clavulanate or a cephalosporin plus a macrolide or doxycycline. The rationale for using broader-spectrum coverage in patients with comorbidities is that such patients likely already have risk factors for antibiotic resistance because of previous healthcare-system contact or use of antibiotic agents, and they are more vulnerable to poor outcomes if inappropriate coverage is part of the initial empirical regimen.4
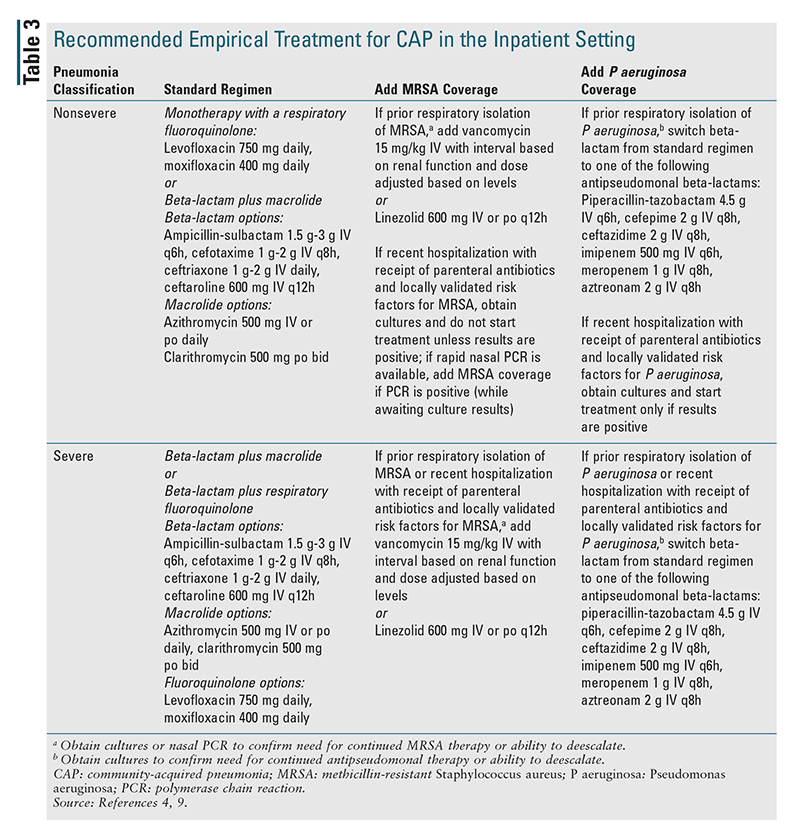
Inpatient Setting: Recommended empirical treatment for CAP in the inpatient setting is given in TABLE 3.4,9 The guidelines delineate different treatment regimens for inpatients with CAP based on whether the patient has severe pneumonia (as defined in TABLE 1), has prior respiratory isolation of MRSA or P aeruginosa (especially within the past year), or has risk factors for these pathogens. The standard recommended empirical regimen for inpatients with nonsevere pneumonia is a beta-lactam plus a macrolide or a respiratory fluoroquinolone alone. In patients in whom the use of both macrolides and fluoroquinolones is contraindicated, a beta-lactam may be used in combination with doxycycline as an alternative empirical regimen. The recommended empirical regimen for inpatients diagnosed with severe pneumonia is combination therapy with a beta-lactam plus a macrolide or a beta-lactam plus a fluoroquinolone. An empirical antimicrobial agent with activity against MRSA and/or P aeruginosa should be added in all inpatients with prior respiratory isolation of the pathogen, as well as in patients having severe pneumonia with recent hospitalization and receipt of parenteral antibiotics (within the past 90 days) in addition to the presence of locally validated risk factors. For patients with nonsevere pneumonia who were recently hospitalized and received parenteral antibiotics (and locally validated risk factors for these pathogens), it is recommended to obtain cultures and to start antibiotics with activity against MRSA and/or P aeruginosa only if culture results are positive. For institutions that have rapid nasal polymerase chain reaction (PCR), therapy against MRSA may be added if PCR results are positive, with therapy adjusted according to the results of cultures. In all cases, antimicrobial therapy may be deescalated after 48 hours (with discontinuation of coverage for MRSA or P aeruginosa) if cultures for these pathogens remain negative.4,9
Although omadacycline, a tetracycline derivative, was approved for treatment of CAP in October 2018, it was not included as an alternative agent in the updated guidelines owing to its lack of efficacy and safety information compared with other agents.4,10
Newly Approved Agents: Lefamulin, the first systemic pleuromutilin antibiotic, was approved by the FDA in August 2019 (after the societies’ approval of the guidelines) for the treatment of adults with CAP caused by S pneumoniae, methicillin-susceptible S aureus (MSSA), H influenzae, Legionella pneumophila, M pneumoniae, and C pneumoniae.11,12 Lefamulin acts as a bacterial protein synthesis inhibitor by targeting the peptidyl transferase center of the 50S bacterial ribosomal subunit. It may be given either IV or orally at a dosage of 150 mg IV every 12 hours or 600 mg orally every 12 hours, with dosage adjustment required for patients with hepatic impairment. Lefamulin may prolong the QT interval, and its use should be avoided in patients who have known QT prolongation or are taking other QT-prolonging agents. Lefamulin has several other drug interactions. Its use should be avoided (because of potential for reduced efficacy) with strong or moderate CYP3A inducers or P-glycoprotein (Pgp) inducers. The oral formulation of lefamulin should not be used with agents that are that are strong CYP3A inhibitors or Pgp inhibitors or with CYP3A4 substrates that prolong the QT interval. Lefamulin may cause fetal harm, and females should be counseled to use effective contraception during treatment and for 2 days after completion of therapy.11-13
Delafloxacin, a fluoroquinolone antibiotic, was approved in October 2019 (after the guidelines were published) for treatment of adults with CAP caused by S pneumoniae, MSSA, selected gram-negative pathogens (Klebsiella pneumoniae, Escherichia coli, P aeruginosa, H influenzae, Haemophilus parainfluenzae), and atypical microorganisms (C pneumoniae, L pneumophila, M pneumoniae).14 It may be given either IV or orally at a dosage of 300 mg IV every 12 hours or 450 mg orally every 12 hours, with adjustment required for patients with severe renal impairment. Delafloxacin has the same warnings and precautions as other agents in the fluoroquinolone antimicrobial class.14,15
Directed Treatment
The patient’s clinical response should be evaluated after initiation of antimicrobial therapy. In cases where blood and/or sputum cultures are recommended, once microbiology culture and sensitivity results are available, antibiotic coverage should be deescalated and therapy should be directed at the pathogen(s) causing disease.4,9
Duration of Therapy
The recommended duration of antibiotic therapy has not changed from previously published guidelines. Patients with CAP should be treated for a minimum of 5 days, with antibiotic therapy continued until the patient achieves clinical stability. Validated measures of clinical stability include resolution of vital sign abnormalities (heart rate, respiratory rate, blood pressure, oxygen saturation, and temperature); ability to eat; and normal mental status. Given that most patients achieve clinical stability within 48 to 72 hours after therapy initiation, a 5-day course typically is sufficient.4 Because of its long half-life and high concentrations in lung tissue, some clinicians administer azithromycin for 3 days (a total of 1.5 g) in patients without pneumonia caused by Legionella.16-18 CAP due to suspected or proven MRSA or P aeruginosa should be treated for 7 days. In CAP patients whose symptoms resolve within 7 days, routine follow-up chest imaging is not recommended.4,9
The Pharmacist’s Role
Pharmacists play an integral role in the management of patients with CAP. Pharmacists should actively work with physicians in both the inpatient and outpatient setting to ensure that patients with pneumonia receive the most appropriate antimicrobial regimen based on patient-specific factors, severity of illness, recent antimicrobial exposure, and risk of drug-resistant pathogens. Pharmacists are also in a key position to recommend deescalation of antimicrobial regimens and to ensure that patients receive the appropriate duration of therapy.
REFERENCES
1. McLaughlin JM, Khan FL, Thoburn EA, et al. Rates of hospitalization for community-acquired pneumonia among US adults: a systematic review. Vaccine. 2020;38(4):741-751.
2. National Center for Health Statistics. Pneumonia. www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed January 22, 2020.
3. Ramirez JA, Wiemken TL, Peyrani P, et al. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis. 2017;65(11):1806-1812.
4. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67.
5. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27-S72.
6. FDA. FDA Drug Safety Communication: azithromycin (Zithromax or Zmax) and the risk of potentially fatal heart rhythms. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-azithromycin-zithromax-or-zmax-and-risk-potentially-fatal-heart. Accessed January 21, 2020.
7. FDA. FDA Drug Safety Communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-updates-warnings-oral-and-injectable-fluoroquinolone-antibiotics. Accessed January 21, 2020.
8. Kim L, McGee L, Tomczyk S, Beall B. Biological and epidemiological features of antibiotic-resistant Streptococcus pneumoniae in pre- and post-conjugate vaccine eras: a United States perspective. Clin Microbiol Rev. 2016;29(3):525-552.
9. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61-e111.
10. FDA. Drug trials snapshots: Nuzyra. www.fda.gov/drugs/drug-trials-snapshots-nuzyra. Accessed January 21, 2020.
11. FDA. FDA approves new antibiotic to treat community-acquired bacterial pneumonia. www.fda.gov/news-events/press-announcements/fda-approves-new-antibiotic-treat-community-acquired-bacterial-pneumonia. Accessed January 21, 2020.
12. Xenleta (lefamulin) package insert. King of Prussia, PA: Nabriva Therapeutics US, Inc; August 2019.
13. Lefamulin (Xenleta) for community-acquired bacterial pneumonia. JAMA. 2019;322(17):1709-1710.
14. Melinta Therapeutics. Melinta Therapeutics announces U.S. FDA approval of supplemental new drug application for Baxdela® (delafloxacin) for the treatment of community-acquired bacterial pneumonia (CABP). http://ir.melinta.com/news-releases/news-release-details/melinta-therapeutics-announces-us-fda-approval-supplemental-new. Accessed January 21, 2020.
15. Baxdela (delafloxacin) package insert. Lincolnshire, IL: Melinta Therapeutics, Inc; October 2019.
16. Pinzone MR, Cacopardo B, Abbo L, Nunnari G. Duration of antimicrobial therapy in community acquired pneumonia: less is more. ScientificWorldJournal. 2014;2014:759138.
17. Mandell LA, File TM Jr. Short-course treatment of community-acquired pneumonia. Clin Infect Dis. 2003;37(6):761-763.
18. File TM. Treatment of community-acquired pneumonia in adults who require hospitalization. UpToDate. Waltham, MA: UpToDate; 2020. www.uptodate.com. Accessed January 21, 2020.
To comment on this article, contact rdavidson@uspharmacist.com.





