US Pharm. 2024;49(8):HS2-HS10.
ABSTRACT: Most preterm infants (defined as those born before a gestational age of 37 weeks) require parenteral nutrition because of gut immaturity or critical illness. The 2023 guideline from the American Society for Parenteral and Enteral Nutrition (ASPEN) offers updated recommendations for premature infants, with a focus on initiation timing, macronutrient dosing, lipid injectable emulsion selection, and adverse event prevention. Although in recent years parenteral nutrition formulations have seen advances that improve safety, uncertainty remains regarding optimization. Pharmacists can play a critical role in implementing the ASPEN guideline in clinical practice to enhance outcomes while simultaneously minimizing adverse effects in this vulnerable patient population.
The World Health Organization defines a preterm infant as a baby born alive before 37 weeks of pregnancy. Preterm infants can be further categorized as moderate-to-late preterm (gestational age 32-37 weeks), very preterm (gestational age 28-<32 weeks), and extremely preterm (gestational age <28 weeks).1 Most preterm infants require parenteral nutrition during the first few days or weeks of life for a variety of reasons, including transient gut immaturity, insufficient progress with enteral feeding, congenital gut disorders, or critical illness.2,3 The duration of parenteral nutrition therapy is based on the indication for parenteral nutrition and the infant’s gestational age.
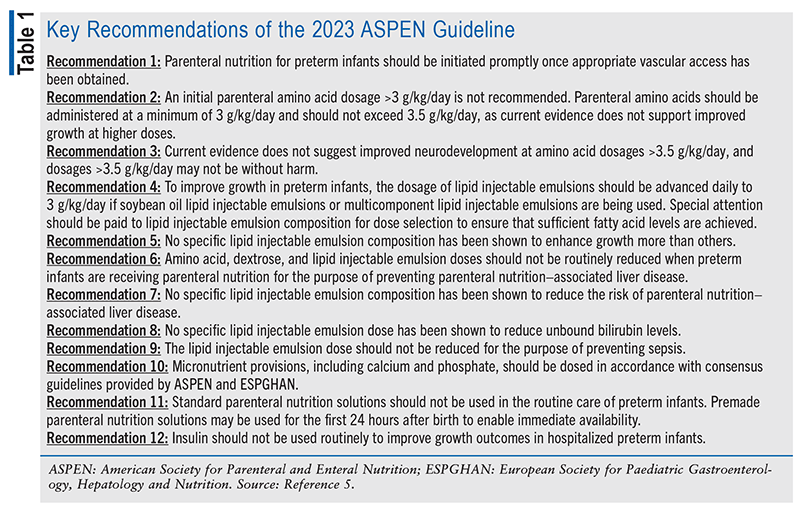
According to the CDC, preterm birth affects approximately one in 10 infants born in the United States.4 For preterm infants, parenteral nutrition is necessary for sustained growth and proper nutrition; however, questions remain on how to lessen the adverse events associated with the use of parenteral nutrition formulations in these patients. To provide further guidance, the American Society for Parenteral and Enteral Nutrition (ASPEN) updated its recommendations in 2023 to address some of the most pressing issues impacting this patient population (TABLE 1).5
Guideline Overview
Over the past several years, many advances have been made in the available parenteral nutrition formulations. This has increased the safety of parenteral nutrition for all patients, but despite these improvements, many unknowns exist regarding the use of these formulations in preterm infants. In its 2023 guideline update, ASPEN evaluated the literature and provided recommendations for the use of parenteral nutrition in preterm infants without a diagnosis of parenteral nutrition–associated liver disease and without a congenital disease that would require surgery.5 These recommendations address key clinical questions regarding the use of parenteral nutrition in preterm infants, including the timing of parenteral nutrition initiation, how macronutrients and micronutrients should be dosed, and considerations when selecting a lipid injectable emulsion.5
The 12 clinical questions posed in the ASPEN guideline yielded 12 corresponding recommendations that for the purposes of this article have been grouped into five areas: 1) parenteral nutrition initiation; 2) amino acid dosing; 3) lipid injectable emulsion selection and dosing; 4) parenteral nutrition–associated liver disease prevention; and 5) other interventions for improving growth outcomes.5 This article will summarize each of these five areas and their associated recommendations.
Parenteral Nutrition Initiation
Controversy surrounds the best time to start parenteral nutrition in preterm infants and what type of parenteral nutrition solution should be used initially. Based on the limited existing evidence, parenteral nutrition should be started once appropriate vascular access is obtained. During the first 24 hours after birth, standardized parenteral nutrition solutions may be used for rapid initiation of parenteral nutrition during those times of day when resources or laboratory values might not be available to provide a customized solution in a timely manner. After the first 24 hours, customized parenteral nutrition solutions should be prepared. Although batch dosing has its advantages, including fewer ordering errors because of its simplicity, patient-specific parenteral nutrition also offers many benefits. Customized solutions form a patient-specific approach that addresses each infant’s metabolic needs. For long-term care, growth and development can be tracked and parenteral nutrition can be adjusted to ensure that the preterm infant continues to follow appropriate growth curves, thus resulting in better outcomes.5
Amino Acid Dosing
Preterm infants receiving parenteral nutrition require adequate amounts and proper formulations of amino acids to meet their protein needs. It has been theorized that increasing the daily dose of amino acids may improve growth and neurodevelopmental outcomes; however, available evidence suggests that there is a limit to the benefits of increased amino acid dosing.5
For both growth and neurodevelopmental outcomes, the recommended maximum target dosage for amino acids is between 3 g/kg/day and 3.5 g/kg/day. In a combined analysis of clinical studies that measured growth in relation to amino acid dosing, there was no significant difference in growth outcomes, including measures such as absolute weight in grams, length for age, and weight for age.5 The dosing ranges of several parenteral nutrition components varied greatly in the studies that evaluated neurodevelopmental outcomes, making it difficult to assess the relationship between amino acid dosing and neurodevelopment. The recommendation to target a dosage between 3 g/kg/day and 3.5 g/kg/day accounts for the protein needs of the infant while exercising caution regarding the potential risks of higher dosages, such as lower Bayley Scales of Infant and Toddler Development score and possible increased rate of cerebral palsy seen in some of the neurodevelopmental evaluations conducted in the studies.5
Lipid Injectable Emulsion Selection and Dosing
The increase in lipid injectable emulsion options in the past several years has brought to light questions regarding the use of these new formulations in preterm infants. In other populations, including older pediatric patients, the use of mixed lipid emulsions rather than soybean oil–only lipid emulsions has been associated with better outcomes, including decreased hospital length of stay.6 However, these results have yet to translate to the preterm-infant population. Potential benefits of using mixed lipid injectable emulsions stem from the possible improved safety profile compared with soybean oil formulations, which have been associated with increased infection susceptibility, pancreatitis, and other health problems in adult and pediatric patients.7 It has also been theorized that the use of certain lipid injectable emulsions could lead to improved growth outcomes. Results of the limited trials available have not produced a clear answer on the benefits of any specific lipid formulation; therefore, ASPEN was unable to make recommendations regarding which product to use to improve growth outcomes, decrease parenteral nutrition–associated liver disease, and reduce unbound bilirubin levels.5
The best lipid injectable emulsion formulation to use is not the only clinical debate surrounding these products; dosing has also raised questions. As with amino acids, it has been theorized that higher lipid injection emulsion doses can lead to improved growth outcomes in preterm infants who require parenteral nutrition. Attention to adequate lipid injectable emulsion dosing is necessary in evaluating the nutritional needs of preterm infants, as proper dosing of these products prevents the development of essential fatty acid deficiency, a condition that leads to poor growth outcomes and other clinically significant adverse effects. To prevent essential fatty acid deficiency, a lipid injectable emulsion dosage of 3 g/kg/day for soybean oil or multicomponent products is recommended. Dosing for fish oil–only products was not mentioned in the guideline. Although the limited evidence available suggests that a higher starting dose may have an increased benefit for growth outcomes, no strong evidence exists for dosages exceeding 3 g/kg/day. Lower doses increase the risk of essential fatty acid deficiency, and caution as well as careful monitoring should always be exercised when dose reductions are being considered in preterm infants.5
All preterm infants are at increased risk for infection—including sepsis—based on an array of factors, including weaker physical barriers (i.e., skin, respiratory and gastrointestinal mucosal barriers) and reduced innate cellular response to pathogens.8 Preterm infants who are unable to receive enteral nutrition possess additional risks because of their lack of exposure to human breast milk, which has been shown to shape the infant’s microbiome and provide additional protection for the gastrointestinal mucosa. The documented risk of increased infections with the use of lipid injectable emulsions led to the question of whether lipid doses should be reduced to prevent sepsis in preterm infants. Only one study was identified that compared low-dose and high-dose lipid injectable emulsions in relation to sepsis, and the results showed no difference between these regimens. Because of the risk of essential fatty acid deficiency with use of lower doses of lipid injectable emulsions, as mentioned above, dose reduction is not recommended for the purpose of preventing sepsis.5
Parenteral Nutrition–Associated Liver Disease Prevention
Long-term use of parenteral nutrition is not without increased risks. Parenteral nutrition–associated liver disease is one of several complications that can occur when enteral nutrition cannot be used over a long period of time. Over the course of treatment, infants receiving parenteral nutrition may develop decreased bile flow in the liver that does not coincide with mechanical obstruction or another type of liver disease.9 As stated previously, no specific lipid injectable emulsion composition has been associated with reduced rates of parenteral nutrition–associated liver disease. Reduced dosing of macronutrients including amino acids, dextrose, and lipid injectable emulsions has been considered as a possible solution to this form of liver disease. Amino acid, dextrose, and lipid injectable emulsion doses should not be routinely reduced when preterm infants are given parenteral nutrition for the purpose of preventing parenteral nutrition–associated liver disease.
Few studies have been conducted to evaluate a dose-based relationship between macronutrients and parenteral nutrition–associated liver disease. To date, no studies have reviewed the effects of the dextrose dose relative to the rate of parenteral nutrition–associated liver disease. In the limited studies on amino acids and lipid injectable emulsions, no significant differences were found between low doses and high doses. Given the risks of lowering the dose of amino acids or lipid injectable emulsions in preterm infants, it is not recommended to reduce the dose of any macronutrient components solely for the purpose of preventing parenteral nutrition–associated liver disease.5
Other Interventions for Improving Growth Outcomes
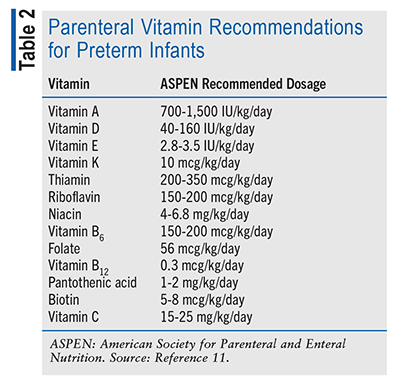
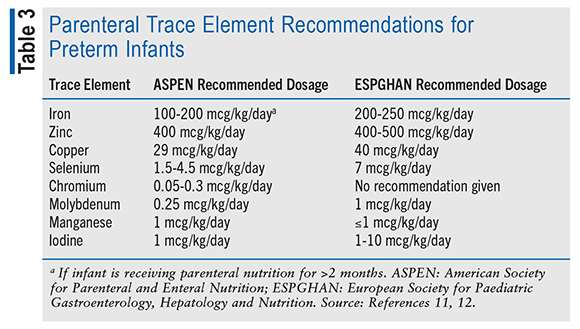
In preterm infants, poor growth shortly after birth has been associated with worsened long-term outcomes, such as in neurodevelopment.10 Therefore, interventions to improve growth outcomes in this population have been widely discussed and sought after. Micronutrients and electrolytes such as calcium, glutamine, manganese, and phosphate have been incorporated into parenteral nutrition to ensure that preterm infants are receiving all of the necessary components for proper development, especially bone development, as preterm infants are at high risk for osteopenia of prematurity. Although the literature does not provide data supporting any specific dosing for improved growth outcomes, ASPEN and the European Society for Paediatric Gastroenterology, Hepatology and Nutrition have included dosing recommendations for parenteral vitamins (TABLE 2) and trace elements (TABLE 3) in preterm infants.11,12
Insulin administration has also been proposed for improving growth outcomes in preterm infants receiving parenteral nutrition. Many preterm infants who receive only or mostly parenteral nutrition shortly after birth experience hyperglycemia. A single study examining the use of insulin for blood glucose maintenance in this population demonstrated a potential decrease in head circumference. Given the lack of evidence of a benefit as well as the risks associated with hypoglycemia, including brain damage and prolonged hospitalization, the routine use of insulin is not recommended in hospitalized preterm infants.13
Conclusion
The use of parenteral nutrition in preterm infants has become safer over the years, but many questions remain on how to improve growth outcomes, minimize adverse events, and ensure proper neurodevelopment. Further investigations into the optimal dosing of both macronutrients and micronutrients for growth development and assessments of the risks and benefits of the various lipid injectable emulsions are among the many areas of research that can help ensure the safe and effective use of parenteral nutrition in preterm infants. Using the current guidance, pharmacists can play a key role in improving both short-term and long-term outcomes in preterm infants who require parenteral nutrition, no matter the duration.
REFERENCES
1. World Health Organization. Preterm birth. May 10, 2023. www.who.int/news-room/fact-sheets/detail/preterm-birth. Accessed May 29, 2024.
2. Darmaun D, Lapillonne A, Simeoni U, et al; Committee on Nutrition of the French Society of Pediatrics (CNSFP), and French Society of Neonatology (SFN). Parenteral nutrition for preterm infants: issues and strategy. Arch Pediatr. 2018;25(4):286-294.
3. National Institute for Health and Care Excellence (NICE). Neonatal Parenteral Nutrition. NICE Guideline. London, England: NICE; 2020.
4. CDC. Preterm birth. May 15, 2024. www.cdc.gov/maternal-infant-health/preterm-birth/index.html. Accessed May 19, 2024.
5. Robinson DT, Calkins KL, Chen Y, et al. Guidelines for parenteral nutrition in preterm infants: the American Society for Parenteral and Enteral Nutrition. JPEN J Parenter Enteral Nutr. 2023;47(7):830-858.
6. Haines KL, Ohnuma T, Hornik CD, et al. Change to mixed-lipid emulsion from soybean oil–based lipid emulsion in pediatric patients. JAMA Netw Open. 2023;6(9):e2332389.
7. Hayes BD, Gosselin S, Calello DP, et al. Systematic review of clinical adverse events reported after acute intravenous lipid emulsion administration. Clin Toxicol (Phila). 2016;54(5):365-404.
8. Collins A, Weitkamp JH, Wynn JL. Why are preterm newborns at increased risk of infection? Arch Dis Child Fetal Neonatal Ed. 2018;103(4):f391-f394.
9. Israelite JC. Pediatric parenteral nutrition-associated liver disease. J Infus Nurs. 2017;40(1):51-54.
10. Ruys CA, van de Lagemaat M, Rotteveel J, et al. Improving long-term health outcomes of preterm infants: how to implement the findings of nutritional intervention studies into daily clinical practice. Eur J Pediatr. 2021;180(6):1665-1673.
11. Vanek VW, Borum P, Buchman A, et al. A.S.P.E.N. position paper: recommendations for changes in commercially available parenteral multivitamin and multi-trace element products. Nutr Clin Pract. 2012;27(4):440-491.
12. Domellöf M, Szitanyi P, Simchowitz V, et al; ESPGHAN/ESPEN/ESPR/CSPEN Working Group on Pediatric Parenteral Nutrition. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: iron and trace minerals. Clin Nutr. 2018;37(6 Pt B):2354-2359.
13. Giouleka S, Gkiouleka M, Tsakiridis I, et al. Diagnosis and management of neonatal hypoglycemia: a comprehensive review of guidelines. Children (Basel). 2023;10(7):1220.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





