US Pharm. 2012;37(7):42-44.
How we think about the etiology and treatment of chronic pain varies considerably depending on the type or description of pain, the history of injury or metabolic disorder, the site of pain, what makes it better or worse, and the age of the patient. In primary care, we frequently determine a vitamin B12 level for patients with neuropathy, and we know that replacing or repleting B12 can potentially reverse neuropathic pain. Similarly, in patients presenting with depression, a thyroid-stimulating hormone (TSH) level is generally determined prior to starting a patient on an antidepressant, as it is well known that abnormal TSH levels can cause various mental health symptoms. As will be discussed in this article, consideration of applicable research literature suggests that vitamin D levels should be determined in patients who present with chronic pain, as there are diverse pain syndromes that seem to occur in the presence of low vitamin D levels.
Vitamin D deficiency in both adults and children has been associated with numerous general health issues including type 1 diabetes, hypertension, metabolic syndrome, ischemic heart disease, falls, broken bones, depression, and cancer.1-3 Recently, vitamin D deficiency has been linked to chronic pain including pain with sickle cell disease, aromatase inhibitor–induced arthralgia, leg pain in older adults, headache, postherpetic neuralgia, pain in patients taking high doses of opioids, symptomatic osteomalacia in gastric bypass patients, diffuse bone and muscle pain, and chronic back pain.4-14 The case reviews and studies presented in this article suggest not only an association of low vitamin D levels with various pain syndromes, but also an improvement in symptoms with vitamin D repletion. Mechanisms underlying this association have not been identified or quantified.
Vitamin D Absorption and Metabolism
The central role of vitamin D is to maintain normal blood levels of calcium and phosphorous, thus helping to prevent osteoporosis and reducing risk of fracture. The absorption and processing of vitamin D is complex, with several influencers. Briefly, with sunlight exposure 7-dehydrocholesterol in the skin is converted to previtamin D3, which is thermally transformed to vitamin D3 (cholecalciferol).2 Vitamin D3 enters the circulation and is metabolized in the liver to 25-hydroxyvitamin D3 (25(OH)D3), which is converted in the kidney to 1, 25(OH)2D3. Serum phosphorus and parathyroid hormone regulate renal production of 1, 25(OH)2D, which regulates calcium metabolism, as this form of vitamin D interacts with its target organs—bone and intestine.2
Standardization of Serum Vitamin D Levels
Official parameters establishing levels of 25-hydroxyvitamin D (25(OH)D) were not established until 2011 (TABLE 1 illustrates current guidelines for determination of normal and abnormal vitamin D levels).15 In November 2010, the Institute of Medicine of the National Academies released a report listing recommended daily allowance of vitamin D assuming minimal sun exposure.15 North Americans need 600 IU daily, with a maximum intake of 4,000 IU/day; however, persons over 70 years of age required 800 IU/day with a maximum of 4,000 IU daily.15 It is not clear at what dose vitamin D becomes toxic. Symptoms of toxicity are due to hypercalcemia, which include nausea, vomiting, muscle weakness, thirst, confusion, QT shortening, sinus tachycardia, and headache.16 Persons with known hypercalcemia should avoid vitamin D supplementation, and those with malabsorption syndrome should be monitored closely for 25(OH)D, calcium, and phosphorus levels.

Repletion Modalities
Vitamin D is most commonly available OTC as cholecalciferol (D3). Ergocalciferol (D2) is available in 50,000 IU capsules and by prescription. Repletion of vitamin D levels requires replacement of serum 25(OH)D to normal levels (i.e., >30 ng/mL). Dosing regimens vary depending on the severity of depletion. For persons with 25(OH)D levels >20 ng/mL, 50,000 IU of D2 weekly for 6 to 8 weeks, followed by at least 800 IU of D3, should be given.17 For persons with 25(OH)D levels between 20 and 30 ng/mL, 800 IU may be sufficient to achieve normal limit threshold of 25(OH)D. Monitoring requires repeat 25(OH)D measurement every 3 to 4 months, with adjustments as needed until levels are normalized.18
Review of Studies
Most of the studies and case reports included in this review were done prior to the recent standards set for determining normal, insufficient, and deficient vitamin D levels. Thus, this review involves patients with various types of chronic pain; vitamin D deficiency is reported with varying definitions of normal versus abnormal vitamin D levels by researchers (TABLE 2).
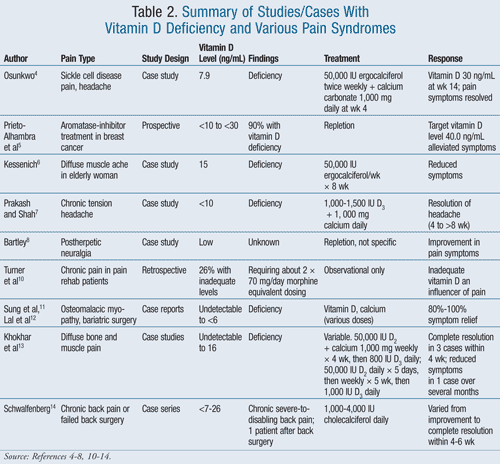
In one case, a 16-year-old premenarcheal female with sickle cell disease presenting with chronic pain, throbbing headaches, nausea, and epigastric pain all unrelieved with nonsteroidal anti-inflammatory drugs (NSAIDs), oral opioids, and other multidisciplinary pain management strategies, was found to have a vitamin D level of 7.9 ng/mL. She was treated with 50,000 IU of vitamin D twice weekly, with 1,000 mg calcium carbonate (TUMS) added at week 4. By week 14, her serum 25(OH)D was 30 ng/mL despite presumed occasional noncompliance, and all pain symptoms were completely resolved.4
Breast cancer patients are often treated with an aromatase inhibitor (AI). Resulting AI-associated arthralgias often lead to nonadherence to therapy. Vitamin D levels were drawn on patients receiving AI treatment (n = 290), and 90% were found to have vitamin D levels <30 ng/mL, including 18.5% with baseline vitamin D levels of <10 ng/mL.5 This study sought to determine a target vitamin D level for alleviation of symptoms, finding that 40 ng/mL or above alleviated symptoms. All participants received 800 IU of D3 with calcium daily. Participants with 25(OH)D levels <30 ng/mL received 800 IU of D3 plus 16,000 IU of D3 orally every 2 weeks. Nothing else was used to treat pain, compliance was not monitored, and it is unknown if patients treated themselves with OTC products. At 3 months, 50% of participants failed to reach adequate vitamin D levels sufficient to alleviate incident pain. Appropriate treatment dosing was determined to be too low, and further study was recommended.5
In another case, an elderly woman complaining of persistent all day and nighttime diffuse muscular aching pain (rated as 6 on a verbal analog scale of 0-10) was unrelieved with OTC medications. Her 25(OH)D level was 15 ng/mL, and she was treated with 50,000 IU of ergocalciferol for 8 weeks, with a significant decline in symptoms.6
Eight patients with tension headache presented with pain described as over the entire head. Those with nearly continuous head pain were found to have 25(OH)D levels of <10 ng/mL.7 When questioned, patients also complained of easy fatigability and pain in the low back, hip, and lower extremities. Conventional headache treatments were not effective. All were treated with 1,000 to 1,500 IU of D3 and 1,000 mg calcium daily, with complete resolution of headache within 4 to 8 weeks. After 3 months, vitamin D levels had normalized, followed by a marked decline in other symptoms.7
Glial inflammation in the central nervous system (CNS) and Schwann cell activation in the peripheral nervous system (PNS) influence pain; activation of the nervi nervorum in the PNS are thought to be influencers of postherpetic neuralgia (PHN).8 Vitamin D can reduce glial inflammation and nitric oxide production from Schwann cell activation, which potentially could influence strategies for reducing PHN pain. Although one patient with PHN and low vitamin D was treated with vitamin D with a reduction in symptoms, vitamin D levels and dosing for repletion were not described. Further studies are needed to determine effectiveness of D repletion in patients affected by PHN.8,9
A retrospective study of 267 chronic pain patients in a pain rehabilitation center found that 26% of patients had inadequate levels of vitamin D. Patients with inadequate vitamin D were taking nearly double the morphine equivalent dose taken by those with normal levels of vitamin D (i.e., approximately 40 ng/day versus approximately 70 mg/day). It was not clear to the authors whether the morphine dose caused low vitamin D levels or if higher doses of opioids were prescribed to treat patients who happened to have low vitamin D levels. Researchers felt that vitamin D inadequacy in this group might represent an underrecognized influencer of nociception and impaired neuromuscular function in chronic pain patients.10
Osteomalacia myopathy characterized by fatigue, muscle aches, bone pain, and muscle weakness is frequently found in patients who have undergone bariatric surgery, including gastric and jejunoileal bypass.11,12 Two case report studies involved patients with histories of either gastric bypass or jejunoileal bypass surgeries who complained of the above symptoms and were found to be deficient in vitamin D; in some cases vitamin D was undetectable. Repletion of vitamin D and calcium over time resulted in symptom resolution.11,12
Several patients were thought to have a metastatic malignancy and underwent imaging and laboratory studies and were eventually found to have vitamin D deficiency. Repletion of vitamin D and calcium over time resulted in symptom resolution.13
A case series of 6 patients with severe or disabling back pain, and one patient with persistent back pain following failed back surgery, were treated with 1,000 to 4,000 IU of cholecalciferol daily. The results varied from improvement to complete resolution of pain within 3 to 6 weeks.14
Summary of Studies
The studies discussed here considered numerous previous investigations of various pain syndromes, their association with vitamin D levels, and treatment with consistent dosing of ergocalciferol and/or cholecalciferol (summarized in TABLE 2.) However, there remains a paucity of current double-blind studies that consider the relationship of these factors. Dosing required for vitamin D repletion based on initial vitamin D levels needs to be investigated and guidelines established. Treatment targets for achieving pain relief in multifarious pain syndromes along with dosing guidelines require further investigation. It is also important to know what types of pain syndromes do not respond to vitamin D treatment. It is assumed that pain from noxious stimuli, such as trauma and surgery, requires immediate relief utilizing typical analgesics. However, further studies are required to explore other pain syndromes that may or may not respond to vitamin D treatment. It seems reasonable for primary care providers to screen for vitamin D levels in their patients who report chronic pain, especially when there is poor response to routine treatment. Similarly, it may be reasonable for pharmacists to consider these relationships in counseling patients who report inadequate or diminished efficacy of pain medications.
REFERENCES
1. Zittermann A. Vitamin D and disease prevention with special reference to cardiovascular disease. Prog Biophy Mol Biol. 2006;92:39-48.
2. Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79:362-371.
3. Vitamin D: a bright spot in nutrition research. Too
little of the sunshine vitamin linked to heart disease, statin-related
muscle pain, other conditions. Harv Heart Lett. 2009;20(4):3.
4. Osunkwo I. Complete resolution of sickle cell chronic pain with high
dose vitamin D therapy: a case report and review of the literature. J Pediatr Hematol Oncol. 2011;33:549-551.
5. Prieto-Alhambra D, Javaid MK, Servitja S, et al.
Vitamin D threshold to prevent aromatase inhibitor-induced arthralgia: a
prospective cohort study. Breast Cancer Res Treat. 2011;125:869-878.
6. Kessenich CR. Vitamin D deficiency and leg pain in the elderly. Nurse Pract. 2010;35:12-13.
7. Prakash S, Shah ND. Chronic tension-type headache with vitamin D deficiency: casual or causal association? Headache. 2009;49:1214-1222.
8. Bartley J. Postherpetic neuralgia, schwann cell activation and vitamin D. Med Hypotheses. 2009;73:927-929.
9. Kiraly SJ, Kiraly MA, Howe RD, Makhani N. Vitamin D as a neuroreactive substance. Scientific World J. 2006;6:125-139.
10. Turner MK, Hooten WM, Schmidt JE, et al. Prevalence
and clinical correlates of vitamin D inadequacy among patients with
chronic pain. Pain Med. 2008;9:979-984.
11. Sung CC, Lee HS, Diang LK, Lin SH. Refractory diffuse bony pain 20 years after jejunoileal bypass. South Med J. 2010;103:570-573.
12. Lal Y, Nair P, Lovrien F, Freeman JW. Osteomalacia presenting as pain syndromes of uncertain etiology. S D Med. 2009;62:197-201.
13. Khokhar JS, Brett AS, Desai A. Vitamin D deficiency masquerading as metastatic cancer: a case series. Am J Med Sci. 2009;337:245-247.
14. Schwalfenberg G. Improvement of chronic back pain or failed back surgery with vitamin D repletion: a case series. J Am Board Fam Med. 2009;22:60-74.
15. Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D.
Washington DC: The National Academies Press; 2011. Brief report.
Released November 20, 2010.
www.iom.edu/Reports/2010/Dietary-Reference-Intakes-for-Calcium-and-Vitamin-D/Report-Brief.aspx.
Accessed April 17, 2012.
16. Lexi-Comp Online (Lexi-Drugs). www.lexi.com. Hudson, OH: Lexi-Comp, Inc. Accessed April 17, 2012.
17. Dawson-Hughes B, Heaney RP, Holick MF, et al. Estimates of optimal vitamin D status. Osteoporosis Int. 2005;16:713-716.
18. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al.
Evaluation, treatment, and prevention of vitamin D deficiency: an
Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930.
To comment on this article, contact rdavidson@uspharmacist.com.





