US Pharm. 2015;40(1):30-33.
ABSTRACT: Obstructive sleep apnea (OSA) is a common chronic breathing disorder associated with reduced quality of life. Given the rising prevalence of obesity, the leading risk factor for this condition, OSA is an important public health concern. Noninvasive continuous positive airway pressure (CPAP) is first-line therapy for OSA. Despite the benefits of consistent CPAP use, adherence remains a challenge. CPAP machines and accessories are sold in community pharmacies and home healthcare settings. It is increasingly important that pharmacists understand the principles of OSA and CPAP therapy to optimize pharmacotherapy with this disorder and reinforce CPAP compliance.
Obstructive sleep apnea (OSA) is a chronic disorder characterized by frequent breathing pauses during sleep. It is a common condition that is associated with significant morbidity and mortality.1,2 The primary risk factor for OSA is obesity, and the incidence of OSA is predicted to rise as the obese population increases.3 A summary of risk factors is provided in TABLE 1.3,4 The gold standard treatment for OSA is continuous positive airway pressure (CPAP) therapy.5 Unfortunately, 30% of patients refuse CPAP therapy from the outset, and 25% discontinue therapy within the first year.6 Here, we summarize OSA, review CPAP therapy, and present pharmacist-implemented strategies to improve outcomes.
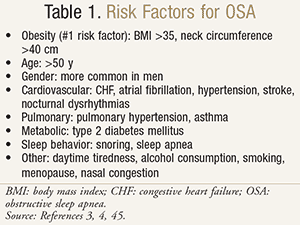
Obstructive Sleep Apnea
The clinical definition of OSA requires sleep-related symptoms (daytime sleepiness, loud snoring, witnessed breathing interruptions, or awakenings due to gasping or choking) with a formal sleep study documenting at least five obstructive respiratory events per hour of sleep. Respiratory events include apneas and hypopneas, or arousals related to respiratory efforts.7 Apnea refers to the complete cessation of breathing and may result from total airway obstruction. Hypopnea is the reduction of breathing defined as at least a 50% reduction in ventilation due to partial airway obstruction.8 OSA is graded according to the apnea-hypopnea index (AHI). The AHI value is calculated by dividing the total number of disordered breathing episodes by the number of hours slept, effectively reducing the sleep study to the number of sleep-related breathing events per hour.
The most serious immediate complication of OSA is motor vehicle accidents due to excessive daytime sleepiness. Patients with OSA can have impaired driving ability akin to that of a person with a blood alcohol level above the legal limit.9 Other possible consequences of OSA include cancer, hypertension, cardiovascular and cerebrovascular diseases, reduced cognitive function, and lipid dysregulation.2,4 Patients with moderate-to-severe OSA are three times more likely to die from cancer and 2.5 times likelier to develop cancer.10 OSA is associated with self-reported heart failure and stroke, systolic hypertension, and increased risk of heart failure and ischemic stroke in men.11-14 Patients with OSA are 2.6 times more likely to experience sudden cardiac death.15
Pathophysiology
The underlying mechanisms whereby OSA contributes to related comorbidities are not fully understood. Obesity narrows the airway. Rapid eye movement sleep relaxes pharyngeal muscles, also leading to airway obstruction. As a result, cessation of airflow, hypoxic events, increased respiratory efforts, and repeated arousals occur. These cyclic changes are associated with the release of various inflammatory mediators. Overall, explanations for the effect of OSA on cancer development are limited by study design. Murine models suggest that intermittent hypoxia can enhance tumor growth and progression.16
OSA has been shown to be associated with atherosclerosis, myocardial infarction, and stroke. The relationship between OSA and coronary atherosclerosis may involve oxidative stress, endothelial dysfunction, and inflammatory processes.17 Patients with OSA but without cardiovascular disease have been shown to have increased microcirculatory oxidant production independent of age, weight, or sex.18 Oxidative stress from the repeated hypoxia and reoxygenation characteristic of OSA can lead to vascular damage and enhanced lipid peroxidation.17,19 In addition, local activation of macrophages and T lymphocytes contributes to inflammation and further tissue injury.17
Continuous Positive Airway Pressure
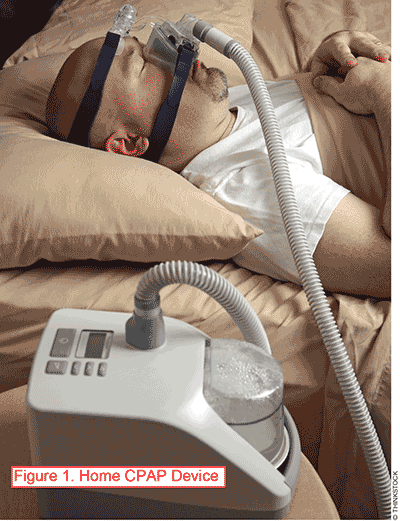
Invasive ventilator support requires an artificial airway and is limited to inpatient use. In the outpatient setting, OSA is commonly treated using noninvasive ventilation via CPAP therapy. Small, bedside CPAP machines (FIGURE 1) are frequently available from community pharmacies and home healthcare companies. Patients will have frequent contact with pharmacists in these settings for medication refills as well as CPAP masks and accessories.
CPAP ventilation provides continuous positive pressure to the airway, acting as a pneumatic splint and maintaining airway patency during sleep. Pressure is delivered using a CPAP machine consisting of three parts: a motor that generates the required pressure, a tube that relays the pressurized air, and a mask with straps that creates the seal between the machine and the patient.20 Based on polysomnography, a patient-specific CPAP pressure is determined and the CPAP mask is custom-fitted. CPAP therapy can be attached to supplemental oxygen to provide up to 6 L/min of oxygen and may be adjusted by a physician.
Benefits
CPAP therapy has been reported to have positive associations with neurobehavioral and cardiovascular outcomes. Randomized placebo-controlled trials have shown that optimal CPAP therapy helps relieve sleepiness and improves vigilance and productivity.21 A meta-analysis evaluating automobile crashes among OSA patients indicated that CPAP therapy alleviated daytime sleepiness after one night of therapy and improved simulated driving performance within 2 to 7 days of treatment.22
CPAP may reduce cardiovascular risk and cardiovascular outcomes (fatal and nonfatal), such as stroke mortality, type 2 diabetes mellitus, hypertension, and cardiovascular mortality in the elderly (TABLE 2).23-38 A greater reduction in mean blood pressure and a stronger dose-response relationship between CPAP use and severity of OSA have been demonstrated.23 The long-term effect of CPAP therapy in nonsleepy OSA patients was shown to be a small decrease in blood pressure.24 This finding contributes to the growing evidence that CPAP therapy improves blood pressure. Among patients with OSA and resistant hypertension, improvements in 24-hour blood pressure and nighttime blood pressure pattern were observed with 12 weeks of CPAP treatment.25 Similarly, a reduced daytime blood pressure was seen in patients with resistant hypertension treated with CPAP and antihypertensive medications compared to blood pressure in patients treated with medications alone.26 Prehypertensive patients may also benefit from CPAP therapy by reductions in both daytime and nighttime blood pressure.27
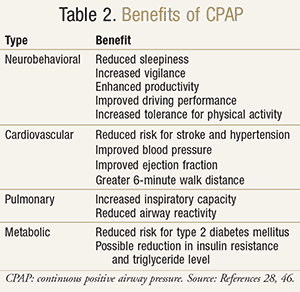
In patients with congestive heart failure (CHF), CPAP therapy may improve ejection fraction, nocturnal oxygenation, 6-minute walk distance, New York Heart Association functional class, blood pressure, and sleepiness, but it has not shown a survival benefit.28-31 Patients with CHF treated with 2 weeks of daily CPAP experienced better pulmonary function and greater tolerance for physical activities, suggesting that CPAP may be an effective adjunct therapy in CHF.32 Similarly, patients with asthma crises have clinically improved pulmonary function with CPAP therapy, and patients with stable asthma have reduced airway reactivity after 7 days of CPAP.33,34 Patients with chronic obstructive pulmonary disease (COPD) who are adherent to their CPAP regimen are reported to have increased inspiratory capacity.35
The benefits of CPAP on blood glucose control have been less clearly delineated. Studies have shown mixed results for improved nighttime blood glucose control in patients with type 2 diabetes mellitus and OSA.36,37 Recently, CPAP therapy combined with weight-loss intervention for 24 weeks produced greater reductions in insulin resistance and serum triglyceride levels than CPAP treatment alone.38
Overcoming Challenges
Adherence, the primary obstacle associated with CPAP therapy, involves patient-specific characteristics, healthcare conditions, and social factors. Challenges to CPAP therapy include mask-related problems (air leakage, skin abrasion, mask discomfort), treatment-related side effects (nasal congestion, dry throat, frequent awakenings), patient attitudes, knowledge, and perceived benefits and risks.5 Poor adherence has been observed in patients who are black, have lower socioeconomic status, or are claustrophobic.39 Smoking, nocturia, and benign prostatic hyperplasia may also complicate adherence to CPAP.40
Role of the Pharmacist
As front-line healthcare professionals, pharmacists can increase OSA awareness and improve adherence with CPAP use.41 Pharmacologic therapy is typically not a primary treatment recommendation for OSA. However, pharmacists are well positioned to counsel on adjunctive medications, such as modafinil. Modafinil is FDA-approved for residual daytime sleepiness despite optimal use of CPAP.42 Drug utilization review focusing on appropriately avoiding benzodiazepines, opioids, and opioid combination products in patients with OSA (these products can worsen carbon dioxide retention and lead to respiratory distress or even require ventilation) who use CPAP can be integrated into a pharmacist’s daily practice. In addition, pharmacists can also promote wellness through education about smoking cessation, exercise maintenance, proper diet and nutrition, effective sleep habits, and compliance with medications and CPAP therapy.
Optimizing CPAP therapy outcomes may be improved using a patient-centered approach. Patients new to CPAP have longer-lasting success if they achieve adherence early. Patients who communicate to healthcare providers any difficulty with autotitrating CPAP use are more likely to remain adherent than those patients who do not report problems.43 Thus, pharmacists who are aware that a patient is initiating CPAP therapy can inquire about the patient’s experiences. For patients who report discomfort with the CPAP device, the pharmacist can help identify the source of discomfort, discuss the importance of a custom fit, and refer the patient to a respiratory therapist or pulmonary medicine specialist.
Patients’ perceived benefit and other psychological variables have stronger associations with CPAP adherence than disease-related factors.44 This suggests that support is integral to successful treatment. Explanations describing the behavioral and cardiovascular benefits of CPAP therapy can help patients understand the purpose of and gains from CPAP treatment.
For patients who report nasal irritation or rhinitis, a common side effect of CPAP therapy, pharmacists can suggest a saline spray or nasal rinses. This strategy is low-risk, relatively inexpensive, safe, and efficacious. Sedative hypnotics (prescribed or OTC) are not generally recommended. In addition, pharmacists can also identify factors associated with poor CPAP adherence.40 For patients seeking smoking cessation support, the pharmacist can recommend follow-up with a physician and educate them about nonprescription agents, such as the nicotine patch, gum, and lozenge. Pharmacists can also discuss prescription medications for smoking cessation.
Summary
OSA is a significant medical and public health problem that is increasingly being recognized. Although CPAP treatment is first-line therapy for OSA, consistent adherence remains a challenge. Strategies to optimize CPAP outcomes involve recognizing patients who use home CPAP, maintaining an open dialogue with them about CPAP therapy, especially during the initiation phase, and helping with side-effect management. Given their access to patients, pharmacists are a well-positioned but underutilized resource to help patients achieve success with CPAP therapy.
REFERENCES
1. Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071-1078.
2. Schwab RJ, Badr SM, Epstein LJ, et al. An official American Thoracic Society statement: continuous positive airway pressure adherence tracking systems. Am J Respir Crit Care Med. 2013;188:613-620.
3. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:1217-1239.
4. Fava C, Dorigonia S, Vedove FD, et al. Effect of CPAP on blood pressure in patients with OSA/hypopnea: a systematic review and meta-analysis. Chest. 2014;145(4):762-771.
5. Wickwire EM, Lettieri CJ, Cairns AA, et al. Maximizing positive airway pressure adherence in adults. Chest. 2013;144: 680-693.
6. Collard P, Pieters T, Aubert G, et al. Compliance with nasal CPAP in obstructive sleep apnea patients. Sleep Med Rev. 1997;1:33-44.
7. Epstein LJ, Kristo D, Strollo PJ, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263-276.
8. Douglas NJ. Chapter 265. Obstructive sleep apnea. In: Longo LD, Fausi AS, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. New York, NY: McGraw Hill; 2012.
9. George CF, Boudrea AC, Smiley A. Simulated driving experience in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1996;154:175-181.
10. Marshall NS, Wong KK, Cullen SR, et al. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton health study cohort. J Clin Sleep Med. 2014;10(4):355-362.
11. Quan SF, Howard BV, Iber C, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20: 1077-1085.
12. Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19-25.
13. Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study: Sleep Heart Health Study. JAMA. 2000;283:1829-1836.
14. Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the Sleep Heart Health Study. Circulation. 2010;122:352-360.
15. Gami AS, Howard DE, Olson EJ, et al. Day-night pattern of sudden cardiac death in obstructive sleep apnea. N Engl J Med. 2005;352:1206-1214.
16. Almendros I, Montserrat JM, Ramirez J, et al. Intermittent hypoxia enhances cancer progression in a mouse model of sleep apnoea. Eur Respir J. 2012;39:215-217.
17. Lui MM, Sau-Man M. OSA and atherosclerosis. J Thorac Dis. 2012;4(2):164-172.
18. Patt BT, Jarjoura D, Haddad DN, et al. Endothelial dysfunction in the microcirculation of patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2010;182(12):1540-1545.
19. Drager LF, Li J, Shin MK, et al. Intermittent hypoxia inhibits clearance of triglyceride-rich lipoproteins and inactivates adipose lipoprotein lipase in a mouse model of sleep apnoea. Eur Heart J. 2012;33:783-790.
20. What is CPAP? National Heart, Lung, and Blood Institute. www.nhlbi.nih.gov/health/health-topics/topics/cpap/. Accessed July 9, 2014.
21. Montserrat JM, Ferrer M, Hernandez L, et al. Effectiveness of CPAP treatment in daytime function in sleep apnea syndrome: a randomized controlled study with an optimized placebo. Am J Respir Crit Care Med. 2001;164:608-613.
22. Tregear S, Reston J, Schoelles K, et al. Continuous positive airway pressure reduces risk of motor vehicle crash among drivers with obstructive sleep apnea: systematic review and meta-analysis. Sleep. 2010;33:1373-1380.
23. Haentjens P, Van Meerhaeghe A, Moscariello A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med. 2007;167:757-764.
24. Barbe F, Duran-Cantollage J, Capote F, et al. Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;181:718-726.
25. Martinez-Garcia MA, Capote F, Campos-Rodriguez F, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA. 2013;310:2407-2415.
26. Pedrosa RP, Drager LF, de Paula LK, et al. Effects of OSA treatment on BP in patients with resistant hypertension: a randomized trial. Chest. 2013;144:1487-1494.
27. Yorgun H, Kabakci, G, Canpolat U, et al. Predictors of blood pressure reduction with nocturnal continuous positive airway pressure therapy in patients with obstructive sleep apnea and prehypertension. Angiology. 2014;65:98-103.
28. Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353:2025-2033.
29. Arzt M, Floras JS, Logan AG, et al. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP). Circulation. 2007;115:3173-3180.
30. Oldenburg O, Bitter T, Wellmann B, et al. Trilevel adaptive servoventilation for the treatment of central and mixed sleep apnea in chronic heart failure patients. Sleep Med. 2013;14:422-427.
31. Ferrier KA, Neill AM, O’Meeghan T, et al. Continuous positive airway pressure in heart failure patients with obstructive sleep apnoea. Intern Med J. 2008;38:829-836.
32. Wittmer VL, Simoes GM, Sogame LC, et al. Effects of continuous positive airway pressure on pulmonary function and exercise tolerance in patients with congestive heart failure. Chest. 2006;130:157-163.
33. Galindo-Filho VC, Brandao DC, Ferreira Rde C, et al. Noninvasive ventilation coupled with nebulization during asthma crisis: a randomized controlled trial. Respir Care. 2013;58:241-249.
34. Busk M, Busk N, Puntenney P, et al. Use of continuous positive airway pressure reduces airway reactivity in adults with asthma. Eur Respir J. 2013;41:317-322.
35. Soares SM, Oliveira RA, Franca SA, et al. Continuous positive airway pressure increases inspiratory capacity of COPD patients. Respirology. 2008;13:387-393.
36. Dawson A, Abel SL, Loving RT, et al. CPAP therapy of obstructive sleep apnea in type 2 diabetics improves glycemic control during sleep. J Clin Sleep Med. 2008;4:538-542.
37. West SD, Nicoll DJ, Wallace TM, et al. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax. 2007;62:969-974.
38. Chirinos JA, Gurubhagavatula I, Teff K, et al. CPAP, weight loss, or both for obstructive sleep apnea. N Engl J Med. 2014;370: 2265-2275.
39. Billings ME, Auckley D, Benca R, et al. Race and residential socioeconomics as predictors of CPAP adherence. Sleep. 2011;34: 1653-1658.
40. Russo-Magno P, O’Brien A, Paciera T, et al. Compliance with CPAP therapy in older men with obstructive sleep apnea. J Am Geriatr Soc. 2001;49:1205-1211.
41. Shoukry G, Wong K, Bartlett D, Saini B. Treatment experience of people with obstructive sleep apnoea seeking continuous positive airways pressure device provision through community pharmacies—a role for pharmacists? Int J Pharm Pract. 2011;19:318-327.
42. Provigil (modafinil) tablet. DailyMed. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=91d2f78d-8501-45d8-b6ff-33b393f75079. Accessed December 5, 2014.
43. Lewis KE, Seale L, Bartle IE, et al. Early predictors of CPAP use for the treatment of obstructive sleep apnea. Sleep. 2004;27: 134-138.
44. Olsen S, Smith S, Oei T, et al. Health belief model predicts adherence to CPAP before experience with CPAP. Eur Respir J. 2008;32:710-717.
45. Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108(5):812-821.
46. Park JG, Ramar K, Olson EJ. Updates on definition, consequences, and management of obstructive sleep apnea. Mayo Clin Proc. 2011; 86(6):549-555.
To comment on this article, contact rdavidson@uspharmacist.com.





