US Pharm. 2015;40(4):20-25.
ABSTRACT: With rising media attention on the increased resistance to antibiotics, many consumers are looking for alternative methods to treat infectious diseases. Recently, probiotics have enjoyed renewed interest as a consumer option, owing to their low cost and a surge in advertisements touting their efficacy. The limited and sometimes inconclusive evidence for probiotics in treating antibiotic-associated diarrhea, vulvovaginal candidiasis, and the common cold restricts the recommendations that pharmacists can make. Currently, there are insufficient data to support an endorsement of the use of probiotic products in all patients. Many studies have demonstrated promising results for a variety of probiotic bacterial strains, but additional standardized clinical research is needed.
A rise in media reporting on the increase in antibiotic resistance has led many consumers to seek innovative and alternative measures to treat infectious diseases. Recently, consumers have had a renewed interest in probiotics as a treatment option. Because of the low cost and an increase in advertising, many people with specific health issues, as well as individuals who simply want to improve their general health, have considered a probiotic-containing product. Many consumers are not fully aware of the various formulations available or do not understand the health benefits of probiotics, and they have turned to pharmacists to answer their questions. However, evidence surrounding the effectiveness of probiotics is lacking, putting pharmacists in a difficult position in terms of counseling patients or recommending a probiotic product from the broad assortment of supplements on the shelf. The purpose of this article is to review the available evidence for the potential use of probiotics as a treatment for three infectious diseases: antimicrobial-associated diarrhea, vulvovaginal candidiasis, and the common cold.
Overview
Probiotics frequently are described as “good bacteria” or as a replacement for native gut bacteria. However, the World Health Organization identifies probiotics as “live microorganisms that, when consumed in adequate amounts as part of food, confer a health benefit on the host.”1 In the United States, probiotics are commonly found in foods—typically dairy products—and dietary supplements. Microorganisms marketed as probiotic agents include species of Lactobacillus and Bifidobacterium (gram-positive, lactic acid–producing bacteria often found in the intestinal tract), although some dietary supplements may contain strains of Enterococcus, Bacillus, Streptococcus, and Escherichia, which are less commonly found in the intestinal tract.2,3
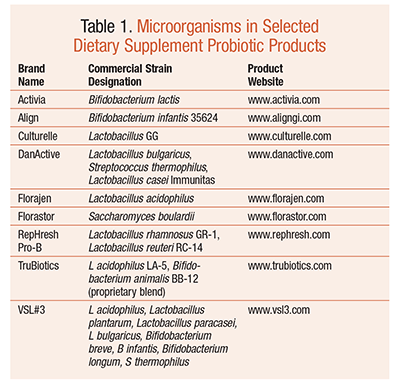
Currently, any product containing probiotics is considered a dietary supplement and is governed by the rules and regulations set forth by the Dietary Supplement Health and Education Act of 1994. This means that the manufacturer can provide only general health claims for the product; it cannot state that any of the ingredients in the product can cure, treat, or prevent a disease. The manufacturer is required by the FDA to follow good manufacturing practices, but it does not have to provide data on the safety or effectiveness of the ingredients in the supplement. This lack of standardization and general labeling is yet another hurdle that pharmacists must negotiate when fielding consumer questions about probiotics. TABLE 1 highlights the probiotic microorganisms found in selected dietary supplement products in the U.S.
Probiotics have been advertised for a variety of conditions, including acute diarrhea, allergies, respiratory infections, irritable bowel syndrome, and inflammatory bowel disease. In addition, potential clinical applications for probiotics currently being researched include colon and bladder cancer, diabetes, and graft-versus-host disease in transplant patients.4
Unlike the situation for many vitamins and minerals, there is no recommended daily dosage for probiotics. The probiotic dosage listed on a dietary supplement product is typically based on a study that has reported a health effect for that type of bacterium. This dosage can vary between different strains of bacteria, as well as by health condition. Specific strains and dosages—if known—for antibiotic-associated diarrhea, vulvovaginal candidiasis, and the common cold are discussed later in this article. Probiotics are sensitive to environmental conditions such as heat, moisture, oxygen, and light. Consumers should take precautions when storing products containing probiotics and follow the instructions indicated on the product label.
Safety of Probiotics
Microorganisms that are “generally regarded as safe” include species of Lactobacillus and Bifidobacterium and certain yeast strains.5 Other bacteria, such as Enterococcus and Streptococcus strains, are not generally regarded as safe, but have been used as probiotics.5 Rarely has a lactic acid–producing bacterium posed a risk to an individual. However, theoretical adverse risks have been raised, including the potential for transmigration, colonization, or transfer of antibiotic resistance.5 These risks, which are speculative, could occur more often in immunocompromised patients, young children, and elderly patients. As such, caution should be exercised in recommending probiotics to these populations. Studies investigating probiotics have been relatively short in duration, limiting the long-term safety data and potential for serious adverse events. To make firm conclusions, more clinical trials investigating the safety of probiotics must be conducted.
Mechanism of Action of Probiotics
A sound gastrointestinal tract is required to maintain good health, but the composition of intestinal flora may be altered when an individual is exposed to an infectious disease. Many consumers believe that taking a probiotic during this time will work by “balancing” the intestinal flora to prevent growth of “bad bacteria.” However, that explanation may be too simplistic to fully describe how a probiotic conveys a health benefit.
It is not fully known how probiotics exert their function or if they have a therapeutic effect on certain health conditions. However, several mechanisms have been proposed to explain how probiotics function.3 According to one theory, probiotics change the composition of intestinal flora.6 This would explain why a health benefit is seen when an individual with vulvovaginal candidiasis takes a product containing lactobacilli, since Lactobacillus strains are typically found in the vagina. Ingestion of the product would theoretically provide more “normal flora” to the area and reduce the growth of the pathogen.
Another proposed mechanism is that probiotics have an effect on the intestinal mucosa.6 It has been shown that some probiotic microorganisms may treat acute diarrhea by stimulating intestinal lactase activity.3 Lactobacillus casei GG, specifically, has been reported to produce an antibacterial substance that inhibits intestinal bacteria, which may explain how the probiotic works in antibiotic-associated diarrhea.3
Probiotics also may work by altering the immune response of the gut.6 Bifidobacterium species and Lactobacillus acidophilus have been particularly noted to have immune effects, but these effects are not always replicated in studies.3
These assumed mechanisms may differ slightly for each bacterial strain. In addition, the dosage, frequency, and route of the probiotic administered may change the activity. The effects seen with probiotics also may vary between individuals.
Use in Antibiotic-Associated Diarrhea
Antibiotic-associated diarrhea is a common complication in the treatment of many infectious diseases. This adverse event may occur soon after commencement of treatment or may be delayed, even until after completion of the antibiotic. The severity of the diarrhea can cause many problems, including electrolyte imbalances and dehydration.
Although the exact cause of antibiotic-associated diarrhea remains unclear, an identified etiology has been linked to bacteria such as Clostridium difficile and Salmonella species. All theories on the cause of antibiotic-associated diarrhea center on the impact of antibiotics on the intestinal system and normal flora, by which opportunistic microorganisms are given the chance to overgrow. Broad-spectrum antibiotics are more commonly implicated in diarrhea, and the occurrence of diarrhea is not tied to dosage or duration.7 Examples of broad-spectrum antibiotics that have been associated with higher rates of antibiotic-associated diarrhea are cephalosporins and penicillins. The treatment of diarrhea not associated with C difficile is usually limited to discontinuation of the antibiotic, along with supportive care.
A yeast, Saccharomyces boulardii, has been used in Europe as an antidiarrheal agent. A double-blind, placebo-controlled study by McFarland and colleagues explored the efficacy of S boulardii in preventing antibiotic-associated diarrhea in patients receiving beta-lactam antibiotics.7 Patients received placebo or S boulardii at a dosage of 3 1010 CFU per day, starting within 72 hours after antibiotic initiation and continuing 3 days after antibiotic discontinuation.7 Nearly 11% of patients, and twice as many placebo patients as S boulardii patients (14 vs. 7, respectively), experienced antibiotic-associated diarrhea. It was concluded that S boulardii decreased the frequency of antibiotic-associated diarrhea by 51% and that if diarrhea did occur, the probiotic significantly shortened the duration.7 Other studies on the use of S boulardii for C difficile infections have been conducted with the probiotic given in conjunction with standard antimicrobial treatment, not allowing for conclusive evidence on the effectiveness of the yeast strain alone.
S boulardii holds promise in decreasing patients’ exposure to antimicrobials, but more research is needed in order to use the probiotic as a single agent for the treatment of antibiotic-associated diarrhea. There also is some evidence that Lactobacillus-based products, whether in the form of yogurt or dietary supplement, may be beneficial against antibiotic-associated diarrhea, but these microorganisms have not been appropriately tested. Thus, a probiotic product cannot be routinely recommended to treat antibiotic-associated diarrhea until more research is conducted.
Use in Vulvovaginal Candidiasis
Vulvovaginal candidiasis, most commonly known as a yeast infection, affects the majority of women at least once in their lifetime. Women who are pregnant, have diabetes, or receive antibiotic or corticosteroid therapy may be at increased risk for the infection.8 A current treatment and prevention method used by many women is the ingestion of yogurt with active cultures of probiotics, mainly Lactobacillus acidophilus. Lactobacilli are commonly found in the vagina, and it is assumed that certain strains may produce inhibitory substances that would protect against the overgrowth of pathogens.
A study by Hilton and colleagues prospectively evaluated whether regular consumption of 8 oz of yogurt containing L acidophilus would decrease the number of incidences of vulvovaginal candidiasis in patients with a history of recurrence.8 Despite potential bias in the study’s unblinded treatment assignment and the unexpected exclusion of controls owing to nonadherence to protocols, ingestion of L acidophilus–containing yogurt led to colonization of the vaginal tract, thereby reducing candidal colonization (control, n = 3.23 ± 2.17, vs. yogurt, n = 0.84 ± 0.90; P = .001) and perhaps preventing infection.8 However, evidence remains limited as to whether yogurt or probiotic dietary supplements can prevent or treat vulvovaginal candidiasis, as many studies are poorly executed, have a small sample size, or lack the necessary steps to provide evidence of effectiveness.
In theory, Lactobacillus strains have produced in vivo inhibitory activity against Candida species, which, if applied directly to the vagina in a suppository form, may be effective.9 Some studies have shown promising results, so a trial of a probiotic containing L acidophilus or Lactobacillus rhamnosus GR-1 in women with frequent recurrence of vulvovaginal candidiasis may be warranted.10 However, more research is necessary before these products can be recommended to all women with vulvovaginal candidiasis.
Use in the Common Cold
The common cold affects adults an average of two to three times per year, according to the National Institute of Allergy and Infectious Diseases.11 In most individuals, a cold lasts about 1 week, but the symptoms can be quite bothersome and disrupt daily activities. Many patients turn to traditional OTC products, such as decongestants or antihistamines, to ease symptoms. Consumers also try other supplements, such as vitamin C, zinc, and echinacea. Probiotics are now investigated as an option for restoring a healthy immune system.
A randomized, double-blind, placebo-controlled study by de Vrese and colleagues investigated whether taking a probiotic product containing three different microorganisms—one Lactobacillus strain and two Bifidobacterium strains—for at least 3 months in the winter/spring period influenced the duration, incidence, and severity of the common cold.12 Both the probiotic supplement and the placebo contained vitamins and minerals, similar to a multivitamin. Although the group taking the probiotic had fewer cold symptoms and a slightly reduced incidence of colds, neither result was statistically significant. However, the probiotic group showed a significant reduction in cold duration (probiotic, 7 ± 0.5 days, vs. placebo, 8.9 ± 1 days; P = .045).12 The significant reduction in cold duration is similar to that seen with supplements such as echinacea, which also has been purported to reduce the duration and severity of a cold.
This study, and many like it, have shown positive effects of probiotics on cold symptoms, especially in terms of reducing the number of days that cold symptoms are experienced. However, many studies, including this one, used a product also containing vitamins and minerals, limiting the conclusiveness of evidence that a probiotic alone is effective for a cold. Also, specific bacterial strains have yet to be identified, thereby limiting routine recommendation of a probiotic as an option to treat cold symptoms.
Discussion
In many studies, probiotics have demonstrated the ability to affect health conditions such as antibiotic-associated diarrhea, vulvovaginal candidiasis, and the common cold. A variety of bacterial species have been studied, including those of Lactobacillus, Bifidobacterium, and Saccharomyces. However, a majority of these studies had significant limitations that made it impossible to determine whether the bacterium was effective for the condition. This may discourage the routine use of probiotics. One particular limitation is the failure to specify the strain of bacteria used in a study, rendering study replication difficult. In addition, many consumer dietary supplements do not include the specific strain or dosage of a probiotic on the label, which makes it hard for the pharmacist to recommend a product, even when a study is appropriately conducted to produce effective results.
Although many clinical trials support the safe use of probiotics, more research is needed to determine the long-term safety of these products. Pharmacists must discuss with patients their need for taking a probiotic and understand their treatment goals in order to properly educate them on the variety of probiotic formulations available. It is equally important for consumers to be aware of the lack of conclusive evidence surrounding these products. Understanding why a patient wishes to take a probiotic will help pharmacists appropriately select a product that contains a bacterial strain that has shown some efficacy.
Conclusion
Data from many studies have shown promising results for a variety of probiotic products in the treatment of a number of infectious diseases. It is important for the pharmacist to understand the patient’s reasons for wanting to use a probiotic product. Until more standardized clinical research is conducted, however, pharmacists are limited in the recommendations they can make to patients.
REFERENCES
1. Food Safety Department, World Health Organization. Modern Food Biotechnology, Human Health and Development: An Evidence-Based Study. Geneva, Switzerland: World Health Organization; 2005.
2. Douglas LC, Sanders ME. Probiotics and prebiotics in dietetics practice. J Am Diet Assoc. 2008;108:510-521.
3. Alvarez-Olmos MI, Oberhelman RA. Probiotic agents and infectious diseases: a modern perspective on a traditional therapy. Clin Infect Dis. 2001;32:1567-1576.
4. Goldin BR, Gorbach SL. Clinical indications for probiotics: an overview. Clin Infect Dis. 2008;46(suppl 2):S96-S100.
5. Snydman DR. The safety of probiotics. Clin Infect Dis. 2008;46(suppl 2):S104-S111.
6. Kotzampassi K, Giamarellos-Bourboulis EJ. Probiotics for infectious diseases: more drugs, less dietary supplementation. Int J Antimicrob Agents. 2012;40:288-296.
7. McFarland LV, Surawicz CM, Greenberg RN, et al. Prevention of B-lactam-associated diarrhea by Saccharomyces boulardii compared with placebo. Am J Gastroenterol. 1995;90:439-448.
8. Hilton E, Isenberg HD, Alperstein P, et al. Ingestion of yogurt containing Lactobacillus acidophilus as prophylaxis for candidal vaginitis. Ann Intern Med. 1992;116:353-357.
9. Elmer GW. Probiotics: “living drugs.” Am J Health Syst Pharm. 2001;58:1101-1109.
10. Falagas ME, Betsi GI, Anthanasiou S. Probiotics for prevention of recurrent vulvovaginal candidiasis: a review. J Antimicrob Chemother. 2006;58:266-272.
11. National Institute of Allergy and Infectious Diseases. Common colds: protect yourself and others. www.niaid.nih.gov/topics/commonCold/Pages/default.aspx. Accessed January 5, 2015.
12. de Vrese M, Winkler P, Rautenberg P, et al. Effect of Lactobacillus gasseri PA 16/8, Bifidobacterium longum SP 07/3, B. bifidum MF 20/5 on common cold episodes: a double-blind, randomized, controlled trial. Clin Nutr. 2005;24:481-491.
To comment on this article, contact rdavidson@uspharmacist.com.





