US Pharm. 2015;40(4):HS19-23.
ABSTRACT: Beta-lactam antibiotics exhibit time-dependent killing. Optimal efficacy with these agents is achieved when free drug concentrations remain above the minimum inhibitory concentration of the infecting organism for an extended duration of the recommended dosing interval. Pharmacokinetic and pharmacodynamic modeling have consistently shown that prolonging the infusion of beta-lactam antibiotics to 3 to 4 hours increases the achievement of this pharmacodynamic goal. The broad-spectrum beta-lactams cefepime, meropenem, and piperacillin-tazobactam are the agents most studied for this dosing strategy, as they have reliable activity against most gram-negative organisms, including Pseudomonas aeruginosa. Data, although limited, suggest superior clinical outcomes with this modality. Health systems are encouraged to explore and adopt this method of dosing.
Multidrug-resistant (MDR) organisms are a growing concern worldwide. Difficulties in infection prevention and control, coupled with the overuse of antimicrobials, have accelerated the rise in incidence of bacterial resistance.1 Infection with these pathogens has been associated with increased hospital length of stay, mortality, and overall healthcare costs.2 The World Health Organization and the Infectious Diseases Society of America have identified MDR organisms as one of the leading obstacles in modern medicine.1,3
Antimicrobial resistance consistently follows closely behind the development of new antimicrobials. The time required for drug discovery, research, and approval far exceeds the time typically observed for the development of resistance following widespread use of an antimicrobial.4 Despite the rise in bacterial resistance, few novel antibiotics have been developed in recent years,5 necessitating optimization of currently available therapies.
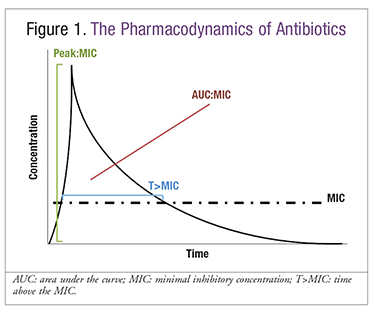
As a class, beta-lactam antibiotics exhibit time-dependent killing.6 Of the three pharmacodynamic parameters described with antimicrobials, optimal efficacy with beta-lactams is achieved when free drug concentrations stay above the minimum inhibitory concentration (MIC) for an extended duration of the recommended dosing interval (FIGURE 1). Prolonged (3-4 hours) or continuous infusion of IV beta-lactams has a higher probability of achieving pharmacodynamic goals compared with standard 30-minute intermittent infusions.7 As the incidence of resistance rises, improvements in pharmacodynamics may allow clinical success even in patients infected with resistant organisms. Cefepime, meropenem, and piperacillin-tazobactam are the beta-lactams best studied for this dosing strategy, as these broad-spectrum agents have reliable activity against most gram-negative organisms, including Pseudomonas aeruginosa. Other beta-lactams have not been studied as extensively, since achievement of pharmacodynamic goals is likely ideal even with standard dosing.
Improving the Probability of Target Attainment
Microbiologic success is dependent on pharmacokinetic and pharmacodynamic parameters and the organism’s MIC.8 As previously mentioned, the pharmacodynamic target for beta-lactam antibiotics is the time the drug concentration remains above the MIC of the infecting organism. More frequent administration of a beta-lactam typically increases this amount of time. For carbapenems, cephalosporins, and penicillins, the optimal percentage of time in the dosing interval in which the drug concentration exceeds the MIC is, respectively, 40%, 60 to 70%, and 50%.7,9,10 As interindividual variability is high, achievement of pharmacodynamic targets by different dosing strategies is often predicted via complex statistical techniques, such as Monte Carlo simulations (MCS).8 In these simulations, the probability of target attainment (PTA; i.e., drug concentration greater than MIC for ≥50% of dosing interval for penicillins) is estimated for a population. Dosing is considered optimal if the PTA is ≥90% in a population.
Prolonged or continuous infusion has consistently resulted in a higher PTA for MICs near or exceeding the susceptibility breakpoint.11-13 In contrast, prolonged or continuous infusion has not yielded improvements in PTA for organisms with low MICs, since standard dosing already achieves these pharmacodynamic targets.11-13 Because most beta-lactams have high renal excretion and renal dysfunction prolongs drug elimination, prolonging drug infusion is unlikely to improve PTA in patients receiving these agents. Prolonged infusion has been shown to have pharmacodynamics similar to continuous infusion, and it may have fewer administration problems, since a dedicated IV line is not required.14 For these reasons, this article will primarily discuss the clinical significance of prolonged-infusion beta-lactam antibiotics in clinical studies.
Dosing Regimens
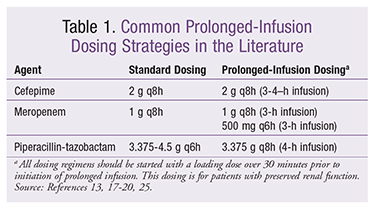
Many different dosing regimens have been employed and studied. Some of the most common dosing regimens are listed in TABLE 1.
Cefepime
MCS with prolonged-infusion (3-4 hours) cefepime have consistently shown pharmacodynamic superiority to standard infusion.12,15 In 2005, Georges and colleagues conducted a small, controlled study that randomized 50 patients with ventilator-associated pneumonia (VAP) to cefepime 2 g every 12 hours by either standard or continuous infusion.16 Clinical outcomes were similar between the groups; however, all but one of the identified pathogens had a low MIC. Based on the organisms’ MICs, the results are not surprising, since standard infusion likely achieves optimal pharmacodynamics in this setting.
In 2013, Bauer and colleagues performed a retrospective, quasi-experimental study of patients with bacteremia or pneumonia caused by susceptible P aeruginosa treated with cefepime 2 g every 8 hours.17 The hospital changed its standard administration to 4-hour prolonged infusion, and outcomes were compared in patients who had received standard infusion prior to the change and patients who received prolonged infusion. Mortality (20% vs. 3%, P = .03) and ICU length of stay (18.5 vs. 8 days, P = .04) were significantly lower after implementation of the prolonged-infusion protocol. Aside from these studies, limited clinical data exist on prolonged-infusion cefepime, although this dosing strategy is being adopted.
Meropenem
As with cefepime, MCS have determined prolonged-infusion meropenem to be pharmacodynamically superior to standard infusion.12,15 Wang compared clinical outcomes for a standard dosing of meropenem 1,000 mg every 8 hours infused over 1 hour versus 500 mg every 6 hours infused over 3 hours in patients with hospital-acquired pneumonia caused by MDR Acinetobacter baumannii.18 Similar outcomes were observed in both groups. No patient developed meropenem-resistant A baumannii, and the significant difference in antibiotic cost proved extended infusion to be an acceptable alternative to standard dosing. A similar study evaluated patients who received 500 mg every 6 hours via standard (30 minutes) or prolonged (3 hours) infusion.19 Lower ventilator days, ICU length of stay, and hospital length of stay were identified in the prolonged-infusion group. Additionally, the prolonged-infusion group exhibited nonsignificantly lower length of antibiotic therapy in the ICU (6.31 vs. 7.16 days) and mortality (12.4% vs. 20.7%).
Fehér and colleagues reviewed 175 patients with severe febrile neutropenia treated with meropenem 1,000 mg every 8 hours infused over 30 minutes or 4 hours (prolonged infusion).20 No difference in 100-day mortality or length of stay was identified; however, clinical cure at 5 days was superior in the prolonged-infusion group (68.4% vs. 40.9%, P <.001). Furthermore, normalization of C-reactive protein (P = .037) and defervescence (P = .021) were higher in the prolonged-infusion group.
Lorente and colleagues conducted a retrospective cohort study evaluating the efficacy of meropenem 1,000 mg every 6 hours as either standard infusion over 30 minutes or continuous infusion for the treatment of gram-negative VAP.21 All 89 patients received once-daily empiric IV tobramycin, and baseline demographics were similar between groups. A significantly greater clinical cure was seen in the continuous-infusion group versus the standard-infusion group when pathogens had MICs of ≥0.5 mcg/mL (80.95% vs. 29.41%, P = .003) or when P aeruginosa was isolated (84.61% vs. 40%, P = .02).
Of note, meropenem has an extremely short stability of approximately 4 hours at room temperature after being mixed in solution.22,23 Hospital pharmacies must be cognizant of this short stability and devise a workflow to ensure that the entire dose is infused within 4 hours of mixing, if prolonged infusion is used. Meropenem has the shortest stability of the antibiotics evaluated in this review.
Piperacillin-Tazobactam
In MCS, a dose of 3.375 g every 8 hours (4-hour infusion) was shown to have a higher PTA than 4.5 g every 6 hours.14,24 In 2007, Lodise and colleagues published a retrospective, quasi-experimental study conducted after implementation of a prolonged-infusion piperacillin-tazobactam protocol in patients infected with P aeruginosa susceptible to piperacillin-tazobactam.13 Outcomes were compared in patients who received 3.375 g every 6 hours (30-minute infusion) and patients who received 3.375 g every 8 hours (4-hour infusion). Fourteen-day all-cause mortality was lower in prolonged-infusion patients (8.8% vs. 15.2%, P >.05), as was median length of hospital stay (18 days vs. 27.5 days, P = .02). In critically ill patients (APACHE II scores ≥17), mortality (12.2% vs. 31.6%, P = .04) and length of stay (21 vs. 38 days, P = .02) were dramatically lower in the prolonged-infusion group despite the lower daily dose.
Yost and Cappelletty conducted a retrospective, multicenter, cohort study comparing prolonged-infusion piperacillin-tazobactam versus nonprolonged-infusion comparator drugs in patients with gram-negative infections.25 Mortality was lower in patients receiving prolonged-infusion piperacillin-tazobactam versus comparators (9.7% vs. 17.9%, P = .02). After confounding variables were controlled for, receipt of prolonged-infusion piperacillin-tazobactam continued to be associated with lower mortality (odds ratio [OR] 0.43, 95% CI 0.18-1.01). When prolonged infusion was compared with standard infusion piperacillin-tazobactam, the difference in mortality was even more pronounced (OR 0.22, 95% CI 0.07-0.76). Four additional studies have shown no major differences in clinical outcomes after changing to reduced-dose, prolonged-infusion piperacillin-tazobactam.26
For patients with preserved renal function (creatinine clearance [CrCl] >20 mL/min) or on continuous renal replacement therapy, a dose of 3.375 g every 8 hours (4-hour infusion) may be considered equivalent to 4.5 g every 6 hours (30-minute infusion). For patients with CrCl <20 mL/min or receiving hemodialysis or peritoneal dialysis, a dose of 3.375 g every 12 hours (4-hour infusion) has been shown to optimize the PTA.9,27
With prolonged-infusion dosing, Y-site incompatibilities should be carefully evaluated, particularly in patients with limited IV line access. Most references list piperacillin-tazobactam and vancomycin as incompatible; however, with prolonged infusion, reduced line concentrations of piperacillin-tazobactam are present. An in vitro study concluded that these reduced concentrations during prolonged infusion are compatible with some concentrations of vancomycin.28
Loading Dose
Since the maximum concentration of drug (Cmax) is lower and the time for maximum concentration of drug (Tmax) is delayed with prolonged infusion, all dosing regimens should be initiated with a loading dose (dose infused over 30 minutes).29,30 For example, piperacillin-tazobactam 4.5 g (30-minute infusion) should be followed in 6 hours by initiation of 3.375 g every 8 hours (4-hour infusion). A loading dose will ensure adequate Cmax and Tmax in severely ill patients. A large retrospective study evaluated clinical outcomes in patients receiving cefepime, meropenem, or piperacillin-tazobactam as either standard or prolonged infusion. No difference in clinical outcomes was identified, and it was hypothesized that the absence of a loading dose may have led to the lack of clinical advantage with prolonged infusion.31
Conclusion
Pharmacokinetic and pharmacodynamic modeling have consistently shown prolonged and continuous infusion to be pharmacodynamically superior to standard infusion of cefepime, meropenem, and piperacillin-tazobactam. Two major strategies exist: 1) use of a lower total daily dose by prolonged infusion, which may result in similar, but possibly improved, clinical outcomes; and 2) use of a similar total daily dose by prolonged infusion, which has a greater chance of resulting in improved clinical outcomes, including decreased mortality. Prolonged infusion has the highest probability of improving clinical outcomes in patients infected with organisms with elevated MICs or virulent pathogens, such as P aeruginosa, and in critically ill patients. Many different dosing regimens have been studied. One factor yet to be adequately researched is the impact of drug penetration into various bodily compartments with the use of prolonged infusion.
Prolonged infusion has been demonstrated to have at least similar—and, in many studies, better—clinical outcomes compared with standard dosing strategies. Hospital systems are encouraged to explore this dosing modality, particularly as rates of bacterial resistance continue to rise.
REFERENCES
1. Boucher HW, Talbot GH, Bradley JS, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1-12.
2. Spellberg B, Bartlett JG, Gilbert DN. The future of antibiotics and resistance. N Engl J Med. 2013;368:299-302.
3. European Centre for Disease Prevention and Control and the European Medicines Agency. The Bacterial Challenge: Time to React. Stockholm, Sweden: European Centre for Disease Prevention and Control; 2009.
4. U.S. Department of Veterans Affairs. How long does the FDA take to approve a drug? www.hiv.va.gov/patient/clinical-trials/drug-approval-process.asp. Accessed December 2, 2014.
5. Infectious Diseases Society of America. Antibiotic development: the 10 ´ ’20 initiative. www.idsociety.org/10x20. Accessed March 18, 2014.
6. Craig WA. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect Dis Clin North Am. 2003;17:479-501.
7. Drusano GL. Prevention of resistance: a goal for dose selection for antimicrobial agents. Clin Infect Dis. 2003;36(suppl 1):S42-S50.
8. Mouton JW, Brown DF, Apfalter P, et al. The role of pharmacokinetics/pharmacodynamics in setting clinical MIC breakpoints: the EUCAST approach. Clin Microbiol Infect. 2012;18:E37-E45.
9. Lodise TP, Lomaestro BM, Drusano GL. Application of antimicrobial pharmacodynamics concepts into clinical practice: focus on beta-lactam antibiotics: insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy. 2006;26:1320-1332.
10. Nicolau DP. Pharmacokinetic and pharmacodynamic properties of meropenem. Clin Infect Dis. 2008;47(suppl 1):S32-S40.
11. Kuti JL, Moss KM, Nicolau DP, Knauft RF. Empiric treatment of multidrug-resistant Burkholderia cepacia lung exacerbation in a patient with cystic fibrosis: application of pharmacodynamic concepts to meropenem therapy. Pharmacotherapy. 2004;24:1641-1645.
12. Kim A, Kuti JL, Nicolau DP. Probability of pharmacodynamic target attainment with standard and prolonged-infusion antibiotic regimens for empiric therapy in adults with hospital-acquired pneumonia. Clin Therapeutics. 2009;31:2765-2778.
13. Lodise TP Jr, Lomaestro B, Drusano GL. Piperacillin-tazobactam for Pseudomonas aeruginosa infection: clinical implications of an extended-infusion dosing strategy. Clin Infect Dis. 2007;44:357-363.
14. Kim A, Sutherland CA, Kuti JL, Nicolau DP. Optimal dosing of piperacillin-tazobactam for the treatment of Pseudomonas aeruginosa infections: prolonged or continuous infusion? Pharmacotherapy. 2007;27:1490-1497.
15. Keel RA, Kuti JL, Sahm DF, Nicolau DP. Pharmacodynamic evaluation of i.v. antimicrobials against Pseudomonas aeruginosa samples collected from U.S. hospitals. Am J Health Syst Pharm. 2011;68:1619-1625.
16. Georges B, Conil JM, Cougot P, et al. Cefepime in critically ill patients: continuous infusion vs. an intermittent dosing regimen. Int J Clin Pharmacol Ther. 2005;43:360-369.
17. Bauer K, West JE, O’Brien JM, Goff DA. Extended-infusion cefepime reduces mortality in patients with Pseudomonas aeruginosa infections. Antimicrob Agents Chemother. 2013;57:2907-2912.
18. Wang D. Experience with extended-infusion meropenem in the management of ventilator-associated pneumonia due to multidrug-resistant Acinetobacter baumannii. Int J Antimicrob Agents. 2009;33:290-291.
19. Dow RJ, Rose WE, Fox BC, Fish JT. Retrospective study of prolonged versus intermittent infusion piperacillin-tazobactam and meropenem in intensive care unit patients at an academic medical center. Infect Dis Clin Pract. 2011;19:413-417.
20. Fehér C, Rovira M, Soriano A, et al. Effect of meropenem administration in extended infusion on the clinical outcome of febrile neutropenia: a retrospective observational study. J Antimicrob Chemother. 2014;69:2556-2562.
21. Lorente L, Lorenzo L, Martín MM, et al. Meropenem by continuous versus intermittent infusion in ventilator-associated pneumonia due to gram-negative bacilli. Ann Pharmacother. 2006;40:219-223.
22. Patel PR, Cook SE. Stability of meropenem in intravenous solutions. Am J Health Syst Pharm. 1997;54:412-421.
23. Merrem (meropenem) product information. Wilmington, DE: AstraZeneca Pharmaceuticals LP; December 2013.
24. Shea KM, Cheatham SC, Smith DW, et al. Comparative pharmacodynamics of intermittent and prolonged infusions of piperacillin/tazobactam using Monte Carlo simulations and steady-state pharmacokinetic data from hospitalized patients. Ann Pharmacother. 2009;43:1747-1754.
25. Yost RJ, Cappelletty DM. The Retrospective Cohort of Extended-Infusion Piperacillin-Tazobactam (RECEIPT) study: a multicenter study. Pharmacotherapy. 2011;31:767-775.
26. Mah GT, Mabasa VH, Chow I, Ensom MH. Evaluating outcomes associated with alternative dosing strategies for piperacillin/tazobactam: a qualitative systematic review. Ann Pharmacother. 2012;46:265-275.
27. Patel N, Scheetz MH, Drusano GL, Lodise TP. Identification of optimal renal dosage adjustments for traditional and extended-infusion piperacillin-tazobactam dosing regimens in hospitalized patients. Antimicrob Agents Chemother. 2010;54:460-465.
28. Leung E, Venkatesan N, Ly SC, Scheetz MH. Physical compatibility of vancomycin and piperacillin sodium-tazobactam at concentrations typically used during prolonged infusions. Am J Health Syst Pharm. 2013;70:1163-1166.
29. Rhodes NJ, MacVane SH, Kuti JL, Scheetz MH. Impact of loading doses on the time to adequate predicted beta-lactam concentrations in prolonged and continuous infusion dosing schemes. Clin Infect Dis. 2014;59:905-907.
30. Roberts JA, Paul SK, Akova M, et al. Reply to Rhodes et al. Clin Infect Dis. 2014;59:907-908.
31. Arnold HM, Hollands JM, Skrupky LP, et al. Prolonged infusion antibiotics for suspected gram-negative infections in the ICU: a before-after study. Ann Pharmacother. 2013;47:170-180.
To comment on this article, contact rdavidson@uspharmacist.com.






