US Pharm. 2024;49(7):HS2-HS10.
ABSTRACT: Chronic obstructive pulmonary disease (COPD) poses a significant global health burden. COPD is characterized by a marked decrease in airflow in the lungs that results in dyspnea and cough. Patients may develop acute exacerbation of COPD (AECOPD), a complication characterized by increased symptom severity. AECOPD diagnosis involves ruling out other potential causes of the symptoms. AECOPD treatment includes antibiotics, corticosteroids, and bronchodilators. Nonpharmacologic treatments not only can help relieve patients’ AECOPD symptoms but may also prevent future exacerbations. Pharmacists are instrumental in the care of patients with AECOPD.
Chronic obstructive pulmonary disease (COPD), which involves inflammation in the small airways and alveoli of the lungs, leads to narrowed airways, reduced elasticity, and increased sputum.1,2 These changes result in symptoms such as dyspnea, cough, and exacerbations. COPD is attributable to multiple factors, including genetics and environmental exposures.1,2 According to the World Health Organization, COPD was the third-highest cause of death worldwide in 2019, and it is the seventh leading cause of disability-adjusted life-years.3 In 2010, the average annual direct healthcare cost for COPD in the United States exceeded $6,000 per patient.4 Furthermore, in 2018, COPD patients in the U.S. lost an average of 5 workdays per year.4
Acute exacerbation of COPD (AECOPD) is a burdensome complication responsible for the lion’s share of COPD’s financial strain on the healthcare system.1,4,5 An exacerbation is defined as an acute increase in dyspnea and/or cough and sputum production.1,6 AECOPD is characterized by increased inflammation, gas trapping, and sputum production, which leads to the marked increase in dyspnea.1,6 Tachypnea and tachycardia may also occur during an exacerbation.1,6
Diagnosis
To diagnose AECOPD, other causes of the patient’s symptoms must be ruled out. Pneumonia, pulmonary embolism, and heart failure exacerbation are the most common conditions that can mimic AECOPD.1 Tests that are helpful for narrowing down the diagnosis include chest radiography, d-dimer levels, and pro–B-type natriuretic peptide levels.1,7,8 The phrasing “exacerbation of respiratory symptoms in patients with COPD” has been suggested to highlight for clinicians the importance of identifying the cause of AECOPD symptoms and pursuing more appropriate treatment options based on the contributing factors.7
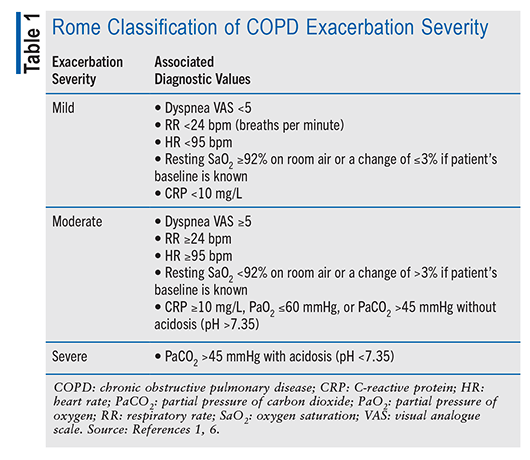
After other causes are ruled out, the severity of the symptoms must be evaluated.1 AECOPD severity is determined by assessing the treatments used after the episode. If short-acting bronchodilators (SABDs) are the only medications used, the AECOPD is classified as mild; moderate AECOPD is treated with SABDs and corticosteroids with or without antibiotics; and severe AECOPD requires treatment in the hospital or emergency department.1 These classifications are limiting because clinicians are using the treatment to define the severity of AECOPD instead of using the severity to define the treatment. AECOPD treatment could be more precise with a new severity-grading system, such as the Rome proposal.6
The Rome proposal uses vital signs and laboratory testing to stratify the severity of COPD exacerbations in both hospital and primary care settings.1,6 This strategy employs a visual analogue scale for dyspnea in addition to respiratory rate, heart rate, and oxygen saturation as the crucial elements for differentiating between mild and moderate exacerbations (TABLE 1).1,6
TREATMENT
Antibiotics
Although antibiotics can be beneficial in some cases of AECOPD, they are not always necessary given that viruses are responsible for most AECOPD occurrences.1 Biomarkers such as procalcitonin and C-reactive protein (CRP) have been studied to consider their role in determining whether to prescribe antibiotics for AECOPD. CRP-guided therapy can reduce antibiotic use in AECOPD without compromising disease-specific quality of life; nonetheless, its routine application is discouraged owing to conflicting data on its clinical utility.1,9 One study of patients with AECOPD and a procalcitonin level <0.1 ng/mL who received either broad-spectrum antibiotics or placebo found no significant differences in treatment-success rate on day 10, length of hospital stay, recurrence of AECOPD in 30 days, intubation, hospital readmission, or mortality.10 However, in a 2020 meta-analysis of randomized, controlled trials (most of them involving inpatients), procalcitonin had no statistically significant effect on duration of antibiotic treatment in AECOPD, and it did not change clinical outcomes such as length of hospital stay or mortality; in fact, the use of procalcitonin-guided therapy led to worse outcomes in ICU patients.11 Based on these conflicting results, it is not currently recommended to use procalcitonin to guide antibiotic treatment in AECOPD.1
Currently, guidelines recommend antibiotics for AECOPD in the following three scenarios: patients who have the three cardinal symptoms of increased sputum volume, sputum purulence, and dyspnea; patients who have two of the three cardinal symptoms, one of these being increased sputum purulence; and patients who are on mechanical ventilation.1
In a retrospective cohort study of mostly hospitalized patients with AECOPD, those who received antibiotics within the first 2 days of admission had significantly lower rates of readmission, need for mechanical ventilation, and inpatient mortality.12 In another study, antibiotic adminstration in ICU patients resulted in reduced mortality and decreases in treatment failure, length of ICU stay, need for additional antibiotics, and duration of mechanical ventilation.13
The bacteria most likely to cause AECOPD are Haemophilus influenzae, Moraxella catarrhalis, Streptococcus pneumoniae, and Pseudomonas aeruginosa.14 Risk factors for P aeruginosa include previous isolation of P aeruginosa, recent hospitalization, systemic corticosteroid use, severe bronchiectasis, and previous antibiotic exposures.15,16 The antibiotics usually used for AECOPD are aminopenicillin with clavulanic acid, macrolides, tetracyclines, or fluoroquinolones.1 Although guidelines do not give specific recommendations on which of these antibiotics is the drug of choice, one pooled analysis showed no significant clinical difference in patients with lower respiratory tract infections (including AECOPD) treated with amoxicillin/amoxicillin-clavulanate versus azithromycin; however, azithromycin was better tolerated.17 The recommended duration of antibiotic use is ≤5 days.1 In two meta-analyses, there was no clinical difference in patients with AECOPD receiving 5 days versus 7 to 10 days of antibiotics, and patients in the longer-duration group had more adverse effects.18,19
Corticosteroids
Glucocorticoids benefit hospitalized AECOPD patients by enhancing oxygenation, improving recovery time, reducing the relapse risk, and shortening the length of hospital stay. Although most hospitalized patients with AECOPD should receive glucocorticoids, emerging evidence indicates that eosinophils may predict the response to glucocorticoids, thereby guiding their use in AECOPD patients.20
Corticosteroids may be administered via inhalation, orally, or IV. No differences have been shown between oral and IV routes in terms of treatment failure, relapse, or mortality, but there might be more adverse drug reactions with parenteral administration.21,22 Parenteral corticosteroids may be considered in patients who did not initially respond to oral corticosteroids or are unable to swallow. High-dose inhaled budesonide was noninferior to systemic corticosteroids regarding changes in forced expiratory volume in 1 second (FEV1); however, it was inferior in terms of oxygenation. High-dose inhaled budesonide may cause less hyperglycemia.23
The recommended oral corticosteroid treatment for AECOPD is prednisone 40 mg daily (or equivalent).1 The Global Initiative for Chronic Obstructive Lung Disease guideline recommends a duration of 5 days, whereas the European Respiratory Society/American Thoracic Society guideline recommends a duration of up to 14 days.1,24 The REDUCE trial demonstrated no difference in time to next exacerbation or recovery time in patients who received 5 days versus 14 days of prednisone 40 mg daily, but the shorter treatment course had the advantage of significantly reduced glucocorticoid exposure.25
Bronchodilators
Short-acting beta2 agonists (SABAs) are a mainstay of therapy in AECOPD.1 The addition of a short-acting muscarinic antagonist (SAMA) to the SABA may be considered. Although the SAMA/SABA combination has demonstrated benefit in stable COPD compared with either agent alone, data on its use in AECOPD are limited.26 Based on its results in stable COPD, however, the combination is commonly used in practice.
These medications are available as metered-dose inhalers, soft mist inhalers, and nebulizers. One systematic review found no difference in FEV1 changes in patients using inhalers versus nebulizers.27 In practice, clinicians may prefer to prescribe the nebulizer because of its ease of use, especially in patients who have difficulty using an inhaler.
Current guidance is to continue the patient’s home inhalers—which may include long-acting muscarinic antagonists and long-acting beta2 agonists—in those experiencing AECOPD.1 These medications bind to the same receptors as their short-acting counterparts and may be considered a duplication of therapy. Continuing a patient’s home inhalers may provide little to no additional benefit while also increasing the risk of possible adverse drug effects and unnecessary drug costs.28 Further research is needed to determine whether there is benefit or harm to continuing a patient’s home bronchodilators during AECOPD.
Respiratory Therapy
Respiratory therapy is an essential part of the treatment of AECOPD. Oxygen saturation should be maintained between 88% and 92%, as this is the safest range for AECOPD.1,29 Blood gases should be monitored frequently during administration of oxygen therapy.1
In severe AECOPD, ventilation support may be required. Noninvasive mechanical ventilation (NIV) is preferred over invasive mechanical ventilation (IMV) for initial ventilation support.1 NIV is indicated in patients experiencing respiratory acidosis, persistent hypoxia, or severe dyspnea.1,30 IMV is indicated for NIV failure or intolerability, diminished consciousness, uncontrolled psychomotor agitation, aspiration/persistent vomiting, hemodynamic instability, or severe ventricular or supraventricular arrhythmia.1
Additional Therapy Considerations
Nonpharmacologic treatments not only can help relieve patients’ AECOPD symptoms but may also prevent future exacerbations. Smoking cessation, vaccinations, and pulmonary-rehabilitation plans should be discussed with the patient before discharge. Data suggest that smoking cessation should be discussed at all points in COPD therapy, including after an AECOPD and at discharge.1,31,32
Influenza, pneumococcal, COVID-19, respiratory syncytial virus (RSV), Tdap (tetanus, diphtheria, pertussis), and shingles vaccines are recommended for eligible patients because they reduce the risk of AECOPD.1,33 PCV20 (pneumococcal conjugate vaccine) alone may be administered at discharge to any COPD patient aged 19 years or older who has not previously received a pneumococcal vaccine.1,31,34 Patients who previously received PPSV23 (pneumococcal polysaccharide vaccine) may get either PCV15 or PCV20 if 1 year or longer has passed since the PPSV23 vaccination. Patients who have received PCV15 should get PPSV23 if 1 year or longer has passed since the PCV15 vaccination.1,34 The RSV vaccine is recommended in COPD patients aged 60 years or older.1,34 It is important to regularly check the CDC’s vaccination guidelines for any updates to the immunization schedule.
Pulmonary rehabilitation consists of education, exercise, and behavioral changes that aim to improve the patient’s physiologic and psychological condition.1 Because pulmonary rehabilitation reduces the risk of hospitalization after recent (≤4 weeks prior) exacerbations, this program should be discussed with the patient before discharge.1,31,32 Pulmonary-rehabilitation programs may be initiated as soon as 1 month after the AECOPD.35 However, it is crucial to account for reduced ventilatory capacity in these patients to avoid precipitating another exacerbation.36
THE PHARMACIST’S ROLE
Pharmacists are instrumental in the care of patients with AECOPD. Counseling these patients on proper inhaler techniques to prevent exacerbations is vital to successful disease control. Pharmacist collaboration with the medical team enables optimization of the medications used to treat a patient’s AECOPD, including the appropriate indication, route, and dosage. In the era of antimicrobial stewardship, pharmacists can ensure the judicious use of antibiotics in patients with AECOPD. Pharmacists can also recommend the implementation of nonpharmacologic therapies, including vaccines.
REFERENCES
1. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2024 Report). Deer Park, IL: Global Initiative for Chronic Obstructive Lung Disease; 2024.
2. Agusti A, Hogg JC. Update on the pathogenesis of chronic obstructive pulmonary disease. N Engl J Med. 2019;381(13):1248-1256.
3. World Health Organization. Global health estimates: life expectancy and leading causes of death and disability. www.who.int/data/gho/data/themes/mortality-and-global-health-estimates. Accessed April 17, 2024.
4. Gutiérrez Villegas C, Paz-Zulueta M, Herrero-Montes M, et al. Cost analysis of chronic obstructive pulmonary disease (COPD): a systematic review. Health Econ Rev. 2021;11(1):31.
5. Iheanacho I, Zhang S, King D, et al. Economic burden of chronic obstructive pulmonary disease (COPD): a systematic literature review. Int J Chron Obstruct Pulmon Dis. 2020;15:439-460.
6. Celli BR, Fabbri LM, Aaron SD, et al. An updated definition and severity classification of chronic obstructive pulmonary disease exacerbations: the Rome proposal. Am J Respir Crit Care Med. 2021;204(11):1251-1258.
7. Beghé B, Verduri A, Roca M, Fabbri LM. Exacerbation of respiratory symptoms in COPD patients may not be exacerbations of COPD. Eur Respir J. 2013;41(4):993-995.
8. Mogelvang R, Goetze JP, Schnohr P, et al. Discriminating between cardiac and pulmonary dysfunction in the general population with dyspnea by plasma pro-B-type natriuretic peptide. J Am Coll Cardiol. 2007;50(17):1694-1707.
9. Butler CC, Gillespie D, White P, et al. C-reactive protein testing to guide antibiotic prescribing for COPD exacerbations. N Engl J Med. 2019;381(2):111-120.
10. Wang JX, Zhang SM, Li XH, et al. Acute exacerbations of chronic obstructive pulmonary disease with low serum procalcitonin values do not benefit from antibiotic treatment: a prospective randomized controlled trial. Int J Infect Dis. 2016;48:40-45.
11. Chen K, Pleasants KA, Pleasants RA, et al. Procalcitonin for antibiotic prescription in chronic obstructive pulmonary disease exacerbations: systematic review, meta-analysis, and clinical perspective. Pulm Ther. 2020;6(2):201-214.
12. Rothberg MB, Pekow PS, Lahti M, et al. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA. 2010;303(20):2035-2042.
13. Nouira S, Marghli S, Belghith M, et al. Once daily oral ofloxacin in chronic obstructive pulmonary disease exacerbation requiring mechanical ventilation: a randomised placebo-controlled trial. Lancet. 2001;358(9298):2020-2025.
14. Bouquet J, Tabor DE, Silver JS, et al. Microbial burden and viral exacerbations in a longitudinal multicenter COPD cohort. Respir Res. 2020;21(1):77.
15. Garcia-Vidal C, Almagro P, Romaní V, et al. Pseudomonas aeruginosa in patients hospitalised for COPD exacerbation: a prospective study. Eur Respir J. 2009;34(5):1072-1078.
16. Gallego M, Pomares X, Espasa M, et al. Pseudomonas aeruginosa isolates in severe chronic obstructive pulmonary disease: characterization and risk factors. BMC Pulm Med. 2014;14:103.
17. Laopaiboon M, Panpanich R, Swa Mya K. Azithromycin for acute lower respiratory tract infections. Cochrane Database Syst Rev. 2015;2015(3):CD001954.
18. Masterton RG, Burley CJ. Randomized, double-blind study comparing 5- and 7-day regimens of oral levofloxacin in patients with acute exacerba tion of chronic bronchitis. Int J Antimicrob Agents. 2001;18(6):503-512.
19. Falagas ME, Avgeri SG, Matthaiou DK, et al. Short- versus long-duration antimicrobial treatment for exacerbations of chronic bronchitis: a meta-analysis. J Antimicrob Chemother. 2008;62(3):442-450.
20. Ramakrishnan S, Jeffers H, Langford-Wiley B, et al. Blood eosinophil-guided oral prednisolone for COPD exacerbations in primary care in the UK (STARR2): a non-inferiority, multicentre, double-blind, placebo-controlled, randomised controlled trial. Lancet Respir Med. 2024;12(1):67-77.
21. Walters JAE, Tan DJ, White CJ, et al. Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;(9):CD001288.
22. de Jong YP, Uil SM, Grotjohan HP, et al. Oral or IV prednisolone in the treatment of COPD exacerbations: a randomized, controlled, double-blind study. Chest. 2007;132(6):1741-1747.
23. Pleasants RA, Wang T, Xu X, et al. Nebulized corticosteroids in the treatment of COPD exacerbations: systematic review, meta-analysis, and clinical perspective. Respir Care. 2018;63(10):1302-1310.
24. Wedzicha JA, Miravitlles M, Hurst JR, et al. Management of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2017;49(3):1600791.
25. Leuppi JD, Schuetz P, Bingisser R, et al. Short-term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA. 2013;309(21):2223-2231.
26. COMBIVENT Inhalation Aerosol Study Group. In chronic obstructive pulmonary disease, a combination of ipratropium and albuterol is more effective than either agent alone: an 85-day multicenter trial. Chest. 1994;105(5):1411-1419.
27. van Geffen WH, Douma WR, Slebos DJ, Kerstjens HAM. Bronchodilators delivered by nebuliser versus pMDI with spacer or DPI for exacerbations of COPD. Cochrane Database Syst Rev. 2016;2016(8):CD011826.
28. Cole JM, Sheehan AH, Jordan JK. Concomitant use of ipratropium and tiotropium in chronic obstructive pulmonary disease. Ann Pharmacother. 2012;46(12):1717-1721.
29. Echevarria C, Steer J, Wason J, Bourke S. Oxygen therapy and inpatient mortality in COPD exacerbation. Emerg Med J. 2021;38(3):170-177.
30. Sellares J, Ferrer M, Anton A, et al. Discontinuing noninvasive ventilation in severe chronic obstructive pulmonary disease exacerbations: a randomised controlled trial. Eur Respir J. 2017;50(1):1601448.
31. Reis AJ, Alves C, Furtado S, et al. COPD exacerbations: management and hospital discharge. Pulmonology. 2018;24(6):345-350.
32. Miravitlles M, Bhutani M, Hurst JR, et al. Implementing an evidence-based COPD hospital discharge protocol: a narrative review and expert recommendations. Adv Ther. 2023;40(10):4236-4263.
33. CDC. Adult immunization schedule by medical condition and other indication. www.cdc.gov/vaccines/schedules/hcp/imz/adult-conditions.html. Accessed April 17, 2024.
34. CDC. Adult immunization schedule notes: pneumococcal vaccination. www.cdc.gov/vaccines/schedules/hcp/imz/adult-schedule-notes.html#note-pneumo. Accessed April 17, 2024.
35. COPD Working Group. Pulmonary rehabilitation for patients with chronic pulmonary disease (COPD): an evidence-based analysis. Ont Health Technol Assess Ser. 2012;12(6):1-75.
36. Troosters T, Janssens W, Demeyer H, Rabinovich RA. Pulmonary rehabilitation and physical interventions. Eur Respir Rev. 2023;32(168):220222.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






